Abstract
Purpose
Doxorubicin/cyclophosphamide followed by docetaxel chemotherapy (AC-D) is an intermediate risk factor (incidence of 10%–20%) for febrile neutropenia (FN) in breast cancer. However, the reported incidence of FN while using this regimen was obtained mostly from Western breast cancer patients, with little data available from Asian patients. This study aimed to assess the incidence of FN in Korean breast cancer patients and to describe clinical variables related to FN.
Methods
From September 2010 to February 2013, data from the Yonsei Cancer Center registry of breast cancer patients who received neoadjuvant or adjuvant chemotherapy with four cycles of AC-D (60 mg/m2 doxorubicin, 600 mg/m2 cyclophosphamide every 3 weeks for four cycles followed by 75 mg/m2 or 100 mg/m2 docetaxel every 3 weeks for four cycles) were analyzed. The incidence of FN, FN associated complications, dose reduction/delays, and relative dose intensity (RDI) were investigated.
Results
Among the 254 patients reported to the registry, the FN incidence after AC-D chemotherapy was 29.5% (75/254), consisting of 25.2% (64/254) events during AC and 4.7% (12/254) during docetaxel chemotherapy. Dose reductions, delays, and RDI less than 85.0% during AC were observed in 16.5% (42/254), 19.5% (47/254), and 11.0% (28/254) of patients, respectively. Patients with FN events frequently experienced dose reduction/delays, which eventually led to a decreased RDI.
The efficacy of myelosuppressive chemotherapy regimens is often restricted by dose-limiting toxicities that can delay subsequent treatment cycles. Febrile neutropenia (FN) is a common adverse effect of chemotherapy, sometimes causing life-threatening complications [1]. Chemotherapy-induced FN may also result in modifications to the chemotherapy dose or schedule, which may compromise treatment efficacy [2]. In breast cancer, there is evidence supporting a close correlation between maintaining the relative dose intensity (RDI) of neoadjuvant or adjuvant chemotherapy and the clinical outcomes of patients [34]. Prevention of chemotherapy-induced FN is therefore a medical priority in neoadjuvant or adjuvant settings.
Recombinant granulocyte-colony stimulating factor (G-CSF) products have emerged as effective therapies for reducing the duration and incidence of chemotherapy-induced neutropenia and FN by stimulating neutrophil proliferation and differentiation in cancer patients [5]. Clinical guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) in the United States, and from the European Organization for Research and Treatment of Cancer (EORTC), all recommend that G-CSF should be administered prophylactically if the risk of FN is greater than 20%. In the case of chemotherapeutic regimens with an intermediate risk of FN (10%–20%), the guidelines emphasize the importance of considering several risk factors for evaluating a patient's overall risk for FN [67]. These risk factors include old age, previous chemotherapy or radiotherapy, pre-existing neutropenia or infection, poor performance status, and poor renal or hepatic functions. However, ethnic or geographic differences in response to the same chemotherapy regimen have so far been poorly investigated.
Sequential doxorubicin/cyclophosphamide and docetaxel (AC-D) is a widely used neoadjuvant and adjuvant chemotherapy regimen for breast cancer. The incidence of FN ranges widely from 3.1% to 25%, and many guidelines including the NCCN, ASCO, and EORTC have categorized this regimen into the intermediate risk group (e.g., the risk of FN is 10%–20%) [89]. However, most of these studies were conducted in Western countries [1011], and there have been few reports on the incidence of FN in Asian countries.
This study was approved by the Institutional Review Board of Yonsei Cancer Center (approval number: 4-2015-1154). Breast cancer patients who received neoadjuvant or adjuvant sequential AC-D from September 2010 to February 2013 were analyzed from the Yonsei Cancer Center registry of breast cancer. Patients with previous exposure to chemotherapeutic agents; inflammatory breast cancer; major cardiovascular, liver, or renal diseases; active infection; inadequate follow-up; or preexisting neutropenia were excluded in order to minimize other confounding factors (Figure 1).
Four cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) followed by four cycles of docetaxel (75 mg/m2 or 100 mg/m2) were administered. Blood samples were collected before each cycle for complete blood cell counts with differential and serum samples for chemistry assays. During the first cycle, nadir blood cell counts were measured between days 10 and 14. After the first cycle, nadir blood cell counts were measured selectively (Figure 2).
Data on patient demographics, pretreatment laboratory parameters, and tumor characteristics were collected. Patients were divided into four subtypes in accordance with the St. Gallen 2011 consensus [16]. The incidence of FN, FN-related hospitalization requiring intravenous antibiotics, FN associated with shock or death, subsequent dose reduction/delay, and other hematologic toxicities according to the common terminology criteria for adverse events (CTCAE), version 4.02, were investigated. FN was defined as neutropenia (<500 neutrophils/µL or <1,000 neutrophils/µL for over 48 hours) with a febrile event (oral temperature ≥38.3℃, or ≥38.0℃ for over 1 hour) observed by medical staff. Dose reduction was defined as reductions in the delivered dosages of agents administered relative to the standard values, and dose delay was defined as a chemotherapy interval of more than 28 days (more than 7 days delay) or early cessation of chemotherapy. RDI was calculated by the method described in Supplementary Table 1 (available online) [17].
All patients received neither G-CSF nor antibiotics as primary prophylaxis for FN. Secondary prophylaxis with G-CSF, antibiotics for FN, and dose reduction/delay were administered at the physicians' discretion. Filgrastim was the most commonly used G-CSF analogue, and choice of antibiotics was based on NCCN guidelines.
Statistical analyses were performed using SPSS version 21.0 for Window (IBM Corp., Armonk, USA). Descriptive statistics were used for baseline characteristics. Binomial two-sided 95% confidence intervals (CIs) for the incidence of FN and dose reduction/delays were calculated. The chi-square test was used for comparison between categorical variables, and the two-sample t-test was used for comparison between continuous variables. Two-sided p-values less than 0.05 were considered statistically significant.
Between September 2010 and February 2013, 254 Korean breast cancer patients receiving AC-D were recruited for the analysis (Table 1). The median patient age was 50 years (range, 27–70 years) and 5.9% (15/254) of patients were more than 65 years old. The mean body weight, body mass index, and body surface area were 59.3±8.5 kg, 23.7±3.4 kg/m2, and 1.61±0.11 m2, respectively. The number of patients with Eastern Cooperative Oncology Group performance status 1 or 2 was 31.5% (80/254). There were 13.4% (34/254) cases of basal-like subtype tumors and 11.4% (29/254) cases of human epidermal growth factor receptor 2 (HER2) enriched subtype tumors. A total of 138 patients received neoadjuvant chemotherapy and 116 patients received adjuvant chemotherapy.
During the AC and D stages of chemotherapy 25.2% (64/254) and 4.7% (12/254) patients experienced FN, respectively (Table 2). Overall, 29.5% of patients experienced FN during chemotherapy (95% CI, 23.9%–35.2%, 75/254). Dose reduction, delays, and RDI less than 85.0% during AC were observed in 16.5% (42/254), 19.5% (47/254), and 11.0% (28/254) of patients, respectively.
The total number of FN events during chemotherapy was 95, including 83 events (87.4%) during AC and 12 events (12.6%) during D (Table 3). Of the 64 patients who experienced FN during AC, 41 patients (64.1%) experienced FN in the first cycle. Of 95 events of FN, 66 events (69.5%) were accompanied by administration of intravenous antibiotics and five (5.3%) were accompanied by septic shock. No chemotherapy-related death was observed. The median duration of admission was 5 days, ranging from 2 to 20 days. β-Lactam/β-lactamase inhibitors were the most frequently used intravenous antibiotics (69.7%) and glycopeptides were used in six events (9.1%). Secondary G-CSF prophylaxis for the next cycle was administered in 58 cases (61.1%). Dose reduction and dose delays were performed in 26.3% and 15.8% of patients, respectively. Fifteen patients experienced more than two events of FN, even with prophylactic G CSF administration, and dose reduction/delays were performed for these patients.
Baseline and treatment characteristics were compared between patients who experienced FN during AC and patients who did not (Table 4). Dose reduction and delay during AC chemotherapy were more frequently observed in patients who experienced FN (39.1% vs. 8.9%, p<0.001; 35.9% vs. 12.6%, p<0.001, respectively). The dose interval during AC was longer in patients who experienced FN (22.3 vs. 21.6 days, p=0.002) and the RDI during AC was lower in patients who experienced FN (90.5% vs. 96.7%, p<0.001). Patients who experienced FN during AC were more likely to have an RDI less than 85.0% (25.0% vs. 6.3%, p<0.001).
We investigated other hematologic toxicities by using the CTCAE grading system (Supplementary Table 2, available online). Patients with FN during AC cycles were more likely to experience grade 3/4 lymphopenia, anemia, and thrombocytopenia (Supplementary Table 3, available online).
This study showed that the incidence of FN during AC-D chemotherapy was 29.5% in breast cancer patients in Korea. FN events occurred frequently during AC chemotherapy (25.2%) and more than half of the FN events (64.1%) occurred in the first cycle. Patients who experienced FN were more likely to have poor performance status and low pretreatment albumin levels, similar to the results of a previous study [18]. Patients with FN events frequently experienced dose reduction/delays, which eventually led to a decreased RDI. Furthermore, we observed frequent hospitalization and use of intravenous antibiotics for the management of FN.
Previous studies reported a diverse range of FN incidence during AC or AC-D chemotherapy. The ECOG 1199 and CALBG 40101 trials reported an incidence of FN of 6.5% and 6.0%, respectively, during adjuvant AC chemotherapy [1920]. The CALGB 9741 and BCIRG-005 trials reported an incidence of 6.0% and 7.7%, respectively, during adjuvant AC-D chemotherapy [1121]. The GEPARDUO study documented an incidence of 3.7% during neoadjuvant AC-D chemotherapy [8]. In contrast to early breast cancer patients, the FN incidence was higher in patients with metastatic breast cancer during AC-D chemotherapy, up to 25% of those without prophylactic G-CSF support [22]. Chan et al. [23] investigated the incidence of FN following AC chemotherapy in Asian countries. After the first cycle, 9.1% (17/189) of patients developed FN and after all cycles this rose to 13.8%. The authors pointed out that the relatively lower incidence of FN in previous randomized control trials could be attributed to patient selection before inclusion into the trial.
Ethnic differences in hematologic toxicity from chemotherapeutic agents or monoclonal antibodies have been described in lung cancer [121324] and renal cell carcinoma patients [14]. In breast cancer, the pharmacokinetics and pharmacodynamics of doxorubicin and docetaxel are different in terms of nadir white blood cell count and neutrophil count between ethnicities [25]. Genetic polymorphisms also influence nadir white blood cell and neutrophil counts in patients receiving cyclophosphamide-based combination chemotherapy [26]. In these previous studies, severe hematologic toxicities were more frequently observed in Asian patients than in Western patients. These findings provide indirect evidence that Asian patients are more likely to experience FN during chemotherapy. Consistent with the findings above, although the patients in our study did not have any of the baseline characteristics that elevate the risk of FN, including other comorbidities, they had FN events more frequently and lower RDIs during AC-D chemotherapy compared to study populations in previous studies that were mostly conducted in Western countries.
Our study has several limitations, including its relatively small sample size, being conducted in a single institution, and its retrospective nature. Indications for admission, selection of antibiotics, secondary G CSF prophylaxis, and dose reduction/delay were according to the individual physicians' judgment rather than protocol-defined management. Additionally, only FN reported officially was counted, and patients' or caregivers' self-documentation of febrile events were excluded, which could imply under-estimation of the incidence of FN. When we included possible cases of FN in the analysis, 32.7% (95% CI, 26.9%–38.5%) and 28.3% (95% CI, 22.8%–33.9%) patients experienced FN during any cycle and during AC cycles, respectively.
In conclusion, the incidence of FN during AC-D in breast cancer patients was 29.5%, 25.2% during AC and 4.7% during D chemotherapy. Patients who experienced FN had more occurrences of hospitalization and dose reduction/delays, which may compromise treatment efficacy and quality of life. Further large prospective studies are required to define the exact incidence of FN in Asian patients with this regimen. In add-ition, whether to consider the use of prophylactic G-CSF in Korean breast cancer patients treated with this regimen needs to be validated in future prospective clinical trials.
ACKNOWLEDGMENTS
The authors would like to thank Dong-Su Jang, a medical illustrator in the Medical Research Support section of Yonsei University College of Medicine, Seoul, Korea, for assistance with the illustrations, and Su Kyoung Park, a medical record administrator in the Analysis Division of Medical Record Team at this institution, for assistance with data management.
References
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006; 106:2258–2266. PMID: 16575919.

2. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007; 25:3158–3167. PMID: 17634496.

3. Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998; 90:1205–1211. PMID: 9719081.
4. Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011; 77:221–240. PMID: 20227889.

5. Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013; 368:1131–1139. PMID: 23514290.

6. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32. PMID: 21095116.

7. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006; 24:3187–3205. PMID: 16682719.

8. von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005; 23:2676–2685. PMID: 15837982.

9. Vriens BE, Aarts MJ, de Vries B, van Gastel SM, Wals J, Smilde TJ, et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer. 2013; 49:3102–3110. PMID: 23850450.

10. Lyman GH, Dale DC, Tomita D, Whittaker S, Crawford J. A retrospective evaluation of chemotherapy dose intensity and supportive care for early-stage breast cancer in a curative setting. Breast Cancer Res Treat. 2013; 139:863–872. PMID: 23771731.

11. Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011; 29:3877–3884. PMID: 21911726.

12. Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009; 27:3540–3546. PMID: 19470925.

13. Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol. 2011; 6:1881–1888. PMID: 21841503.

14. Wang Y, Choueiri TK, Lee JL, Tan MH, Rha SY, North SA, et al. Anti-VEGF therapy in mRCC: differences between Asian and non-Asian patients. Br J Cancer. 2014; 110:1433–1437. PMID: 24548864.

15. Huang RS, Kistner EO, Bleibel WK, Shukla SJ, Dolan ME. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol Cancer Ther. 2007; 6:31–36. PMID: 17237264.

16. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes: dealing with the diversity of breast cancer. Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747. PMID: 21709140.
17. Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012; 133:301–310. PMID: 22270932.

18. Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014; 90:190–199. PMID: 24434034.

19. Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O'Regan R, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol. 2014; 32:2311–2317. PMID: 24934787.

20. Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008; 358:1663–1671. PMID: 18420499.

21. Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003; 21:1431–1439. PMID: 12668651.

22. Perez EA, Geeraerts L, Suman VJ, Adjei AA, Baron AT, Hatfield AK, et al. A randomized phase II study of sequential docetaxel and doxorubicin/cyclophosphamide in patients with metastatic breast cancer. Ann Oncol. 2002; 13:1225–1235. PMID: 12181246.

23. Chan A, Chen C, Chiang J, Tan SH, Ng R. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer. 2012; 20:1525–1532. PMID: 21818641.

24. Soo RA, Wang LZ, Ng SS, Chong PY, Yong WP, Lee SC, et al. Distribution of gemcitabine pathway genotypes in ethnic Asians and their association with outcome in non-small cell lung cancer patients. Lung Cancer. 2009; 63:121–127. PMID: 18538445.

25. Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008; 8:139–146. PMID: 17876342.
26. Haroun F, Al-Shaar L, Habib RH, El-Saghir N, Tfayli A, Bazarbachi A, et al. Effects of CYP2B6 genetic polymorphisms in patients receiving cyclophosphamide combination chemotherapy for breast cancer. Cancer Chemother Pharmacol. 2015; 75:207–214. PMID: 25428516.

Supplementary Materials
Supplementary Table 2
Rates of treatment related hematologic toxicities in all patients (total no. of patients=254)
Supplementary Table 3
Comparison of grade 3/4 hematologic toxicity by experience of febrile neutropenia during doxorubicin/cyclophosphamide (experience of febrile neutropenia versus no experience of febrile neutropenia during doxorubicin/cyclophosphamide)
Figure 1
The consort diagram shows the patient inclusion and exclusion criteria.
AC=doxorubicin/cyclophosphamide; D=docetaxel.
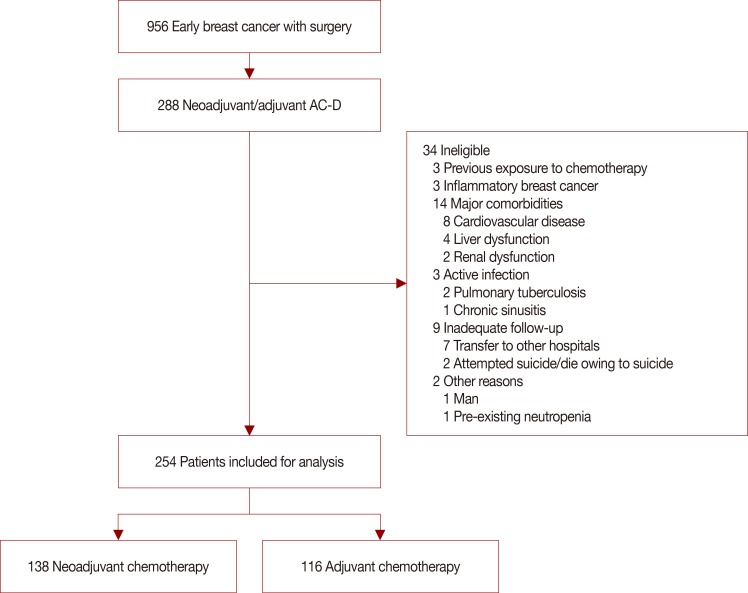
Figure 2
The treatment schema shows the regimen schedule.
IVH=intravenous bolus; IVF=intravenous infusion; CBC=complete blood cell count; SMA=serum metabolic analysis.
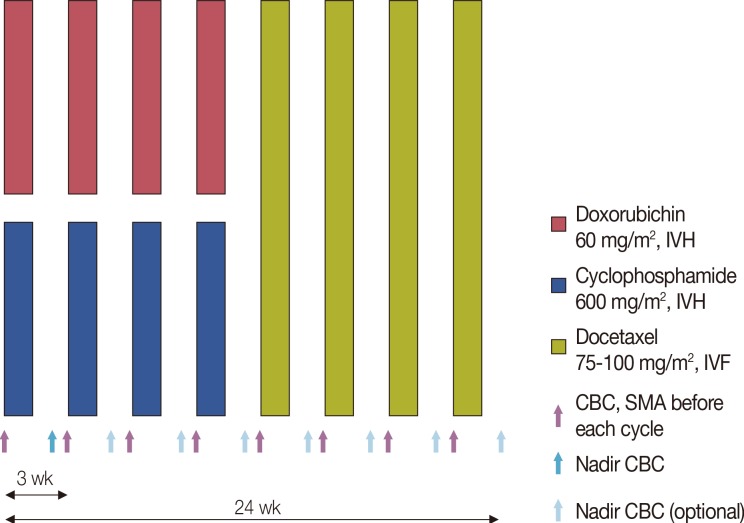
Table 1
Baseline characteristics

Table 2
Incidence of febrile neutropenia, dose reduction, dose delay, and relative dose intensity <85.0% during doxorubicin/cyclophosphamide
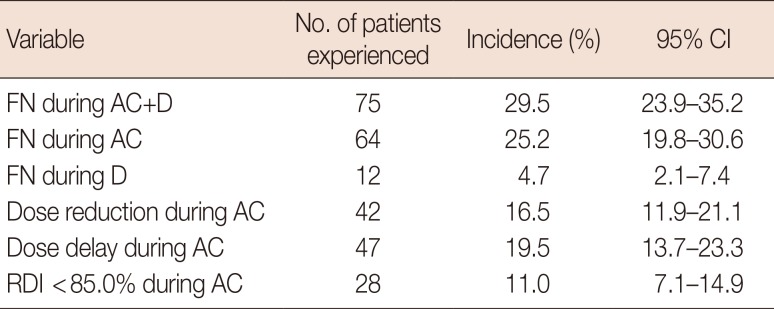
Table 3
Description of febrile neutropenia* (total no. of events=95, total no. of patients=75)
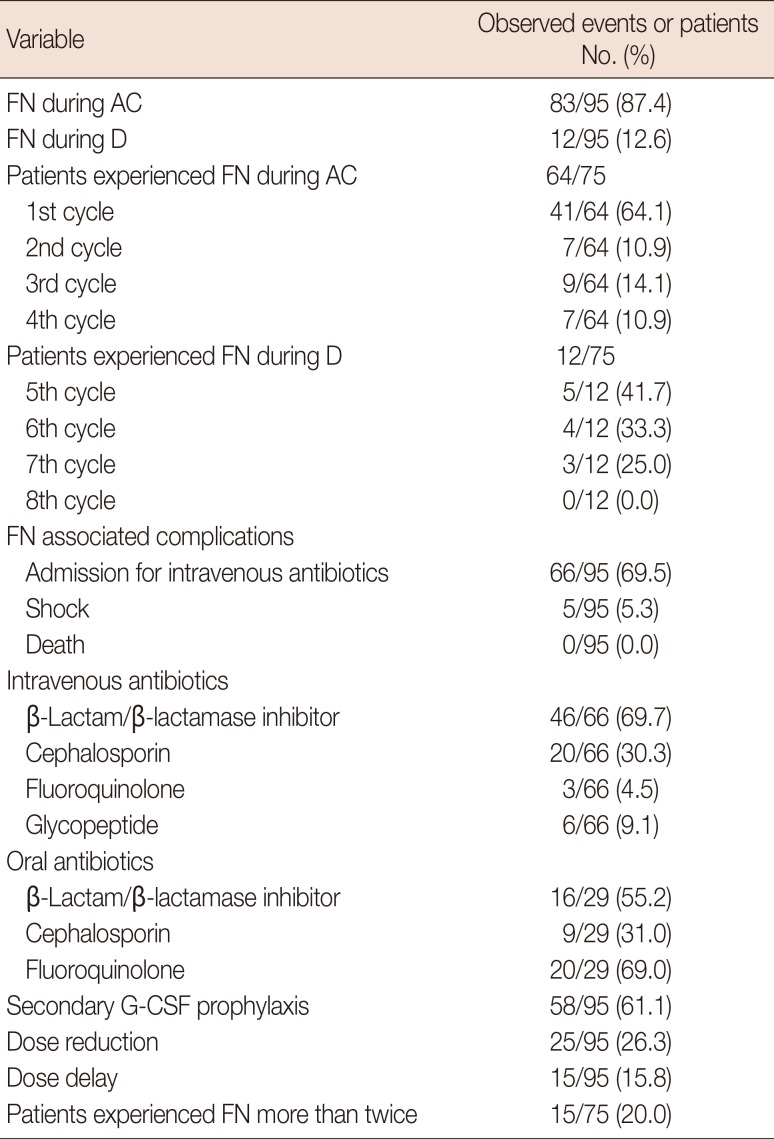
Table 4
Comparison of patient and treatment characteristics by experience of febrile neutropenia during doxorubicin/cyclophosphamide (experience of febrile neutropenia versus no experience of febrile neutropenia during doxorubicin/cyclophosphamide)
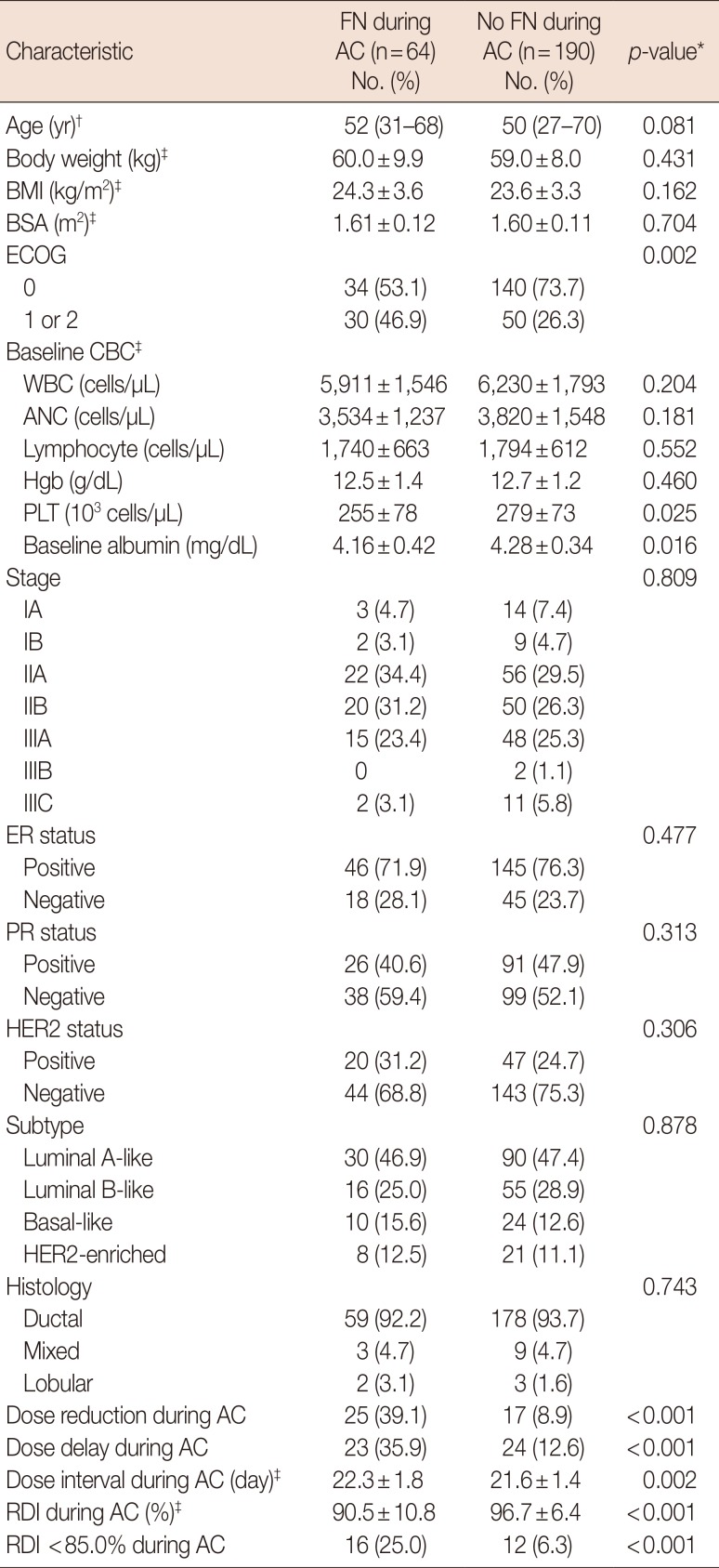
FN=febrile neutropenia; AC=doxorubicin/cyclophosphamide; BMI=body mass index; BSA=body surface area; ECOG=Eastern Cooperative Oncology Group; CBC=complete blood cell count; WBC=white blood cell count; ANC=absolute neutrophil count; Hgb=hemoglobin; PLT=platelet; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; RDI=relative dose intensity.
*Comparison between patients who experienced febrile neutropenia and those who did not; †Expressed as median (range); ‡Expressed as mean±SD.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download