Abstract
Purpose
Breast cancer is an important cause of death among women. The development of radioresistance in breast cancer leads to recurrence after radiotherapy. Caffeic acid phenethyl ester (CAPE), a polyphenolic compound of honeybee propolis, is known to have anticancer properties. In this study, we examined whether CAPE enhanced the radiation sensitivity of MDA-MB-231 (estrogen receptor-negative) and T47D (estrogen receptor-positive) cell lines.
Methods
The cytotoxic effect of CAPE on MDA-MB-231 and T47D breast cancer cells was evaluated by performing an 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay. To assess clonogenic ability, MDA-MB-231 and T47D cells were treated with CAPE (1 µM) for 72 hours before irradiation, and then, a colony assay was performed. A comet assay was used to determine the number of DNA strand breaks at four different times.
Results
CAPE decreased the viability of both cell lines in a dose- and time-dependent manner. In the clonogenic assay, pretreatment of cells with CAPE before irradiation significantly reduced the surviving fraction of MDA-MB-231 cells at doses of 6 and 8 Gy. A reduction in the surviving fraction of T47D cells was observed relative to MDA-MB-231 at lower doses of radiation. Additionally, CAPE maintained radiation-induced DNA damage in T47D cells for a longer period than in MDA-MB-231 cells.
Breast cancer is quite prevalent and the leading cause of cancer-related death among women worldwide. Additionally, breast cancer has been investigated as a heterogeneous malignancy with various responses to therapy [1]. Particularly, radiotherapy is an effective treatment for breast cancer [2], but increasing damage to normal tissues and radioresistance in tumor cells are the two main limitations of radiotherapy treatment [3]. The development of radioresistance leads to the recurrence of more aggressive phenotypes in patients [4]. Previous studies have shown that the use of drugs in combination with radiation leads to enhanced radiation sensitivity by overcoming radioresistance and minimizing damage to normal tissues. Hence, there is growing interest to identify new radiosensitizing agents.
Recent studies have indicated that natural polyphenols can enhance radiation-induced cell killing with minimal toxicity to normal cells [5]. Polyphenols are a group of common substances present in most plants and possess diverse biological features. They play an important role in suppressing carcinogenesis by targeting proteins involved in cell signaling pathways [6].
Caffeic acid phenethyl ester (CAPE) is the main polyphenol extracted from honeybee propolis. CAPE exhibits many interesting properties, including antiviral, anti-inflammatory, and antioxidant effects [7]. CAPE is considered to have anticancer effects in different tumor cell lines; however, it shows differential cytotoxic activity against tumor and surrounding normal cells [89]. Several studies have reported a role for CAPE in apoptosis and cell cycle arrest [10]. CAPE is also known to specifically inhibit the nuclear factor κB (NF-κB) cell signaling pathway, which is involved in resistance to radiotherapy [1112].
DNA is thought to be the main target of ionizing radiation in cells. Double-strand breaks are the main form of damage resulting in cell death after irradiation. Activation of DNA repair pathways is correlated with resistance in cancer cells and leads to therapeutic failure [13]. Some radiosensitizers may enhance the radiation response by prolonging DNA damage and impairing DNA repair mechanisms [14]. Therefore, agents that affect this mechanism should be identified and evaluated.
The radiosensitization effect of CAPE in the treatment of breast cancer cell lines is unclear. In this study, we compared the combined effect of CAPE and ionizing radiation in MDA-MB-231 (estrogen receptor-negative) and T47D (estrogen receptor-positive) breast cancer cell lines by analyzing their clonogenic abilities. We also determined whether CAPE radiosensitizes breast cancer by prolonging radiation-induced strand breaks.
The receptor-negative and receptor-positive (MDA-MB-231 and T47D) cells were obtained from the National Cell Bank of Iran (Pastuer Institute, Tehran, Iran). The cells were cultured in Roswell Park Memorial Institute medium (RPMI-1640; Gibco, Grand Island, USA) containing penicillin (Sigma-Aldrich, St. Louis, USA) and streptomycin (Jaber Ibn-Hayan, Tehran, Iran) and supplemented with 10% fetal bovine serum (Gibco) at 37℃ in a 98% humidified atmosphere with 5% CO2. CAPE was purchased from Tocris Bioscience (Bristol, UK). All chemicals were obtained from Merck Company (Darmstadt, Germany) unless otherwise mentioned.
MDA-MB-231 and T47D cells were seeded at densities of 3,000 and 6,000 cells/well in 96-well plates (Nunc, Roskilde, Denmark) for 24 and 48 hours, respectively, to reach log phase. Next, the cells were treated with five doses of CAPE (1, 5, 10, 20, 60, and 100 µM) and incubated for 24, 48, and 72 hours. Subsequently, 100 µL MTT solution (Sigma-Aldrich) at a concentration of 0.5 mg/mL was added and the cells were incubated for 3 hours followed by the addition of 100 µL dimethyl sulfoxide to dissolve the formazan crystals. Absorbance at 570 nm and 630 nm was measured using a Microplate Spectrophotometer (BioTek, Winooski, USA). The difference in absorbance between the two wavelengths was measured and the percentage of viable cells was calculated as (absorbance of sample/absorbance of control)×100 for each concentration of CAPE.
Cultured MDA-MB-231cells and T47D at 10,000 and 20,000 cells/cm2 densities were treated with 1, 5, and 10 µM CAPE for 72 hours. Next, 3,000 and 500 MDA-MB-231 and T47D cells were re-plated into 60- and 35-mm petri dishes, respectively. Plates were maintained in fresh culture medium containing 10% fetal bovine serum for 7 days (MDA-MB-231) and 12 days (T47D). Finally, colonies were fixed with 2% formaldehyde solution, stained with 5% crystal violet dye, and colonies containing more than 50 cells were counted.
Cells in log phase were seeded into T12.5 culture flasks (Jet Bio-Filtration, Guangzhou, China) and incubated for 24 and 48 hours for MDA-MB-231 and T47D cells, respectively. Next, the cells were treated with 1 µM CAPE for 72 hours. For irradiation, drug-containing medium was removed and fresh medium was added to each flask and then the cells were exposed to 2, 4, 6, and 8 Gy. Irradiation was performed using a 6 MeV X-ray photon, produced by a linear accelerator (Siemens Primus; Siemens AG, Erlangen, Germany) at a dose rate 200 cGy/min. Control and CAPE alone groups were not exposed to radiation.
A colony assay was performed as described previously [15]. Cells were counted and appropriate numbers of MDA-MB-231 and T47D cells were plated into 60- and 35-mm petri dishes (Nunc), respectively. To obtain a countable number of colonies, the number of cells plated was increased depending on the radiation dose. After incubation of MDA-MB-231 and T47D cells at 37℃ for 7 and 12 days, the colonies were fixed, stained, and counted. The surviving fraction was calculated as the number of colonies/(number of cells×control plating efficiency) in three independent experiments. A linear-quadratic model was used to fit the survival curves.
A comet assay was performed to determine the level of DNA damage as described by Rouhani et al. [16] MDA-MB-231 and T47D cells were cultured in T12.5 flasks and incubated for 24 and 48 hours to reach log phase, respectively. Each experiment included four groups: control group (no treatment but incubated in fresh medium for additional 72 hours), CAPE-treated group (treated with CAPE for 72 hours), irradiated group (incubated in fresh medium for additional 72 hours, then exposed to radiation), CAPE plus radiation group (treated with CAPE for 72 hours, then irradiated). After incubation, the cells were exposed to radiation (6 Gy). The control and CAPE-treated groups were not exposed to radiation. Immediately after irradiation, the cells were trypsinized, and cell suspensions were prepared. Next, 30,000 cells were mixed with 1% (g/mL) low-melting point agarose (Sigma-Aldrich) and fixed on microscope slides precoated with a layer of normal agarose (1% g/mL). After lysis and denaturation, electrophoresis was conducted under alkaline conditions. Neutralization buffer was added and the gel was stained with 20 µg/mL ethidium bromide. A fluorescence microscope (Axioskop 2 plus; Zeiss, Jena, Germany) was used to acquire images of each slide. At least 50 comet images (up to 400) were analyzed for each group at four different times (0, 30, 60, and 120 minutes) after irradiation using Comet Score 1.5 software (TriTek Corp., Sumerduck, USA). Mean values of %DNA in the tail and Olive tail moment are reported from two independent experiments. Synergy was determined based on comparison of observed (experimental) and expected amounts of %DNA in the tail. As previously described, the effect of each treatment was calculated separately and the sum of these values was represented as the expected effect (Exp). The result of combined treatment was considered the observed value (Obs). If (Obs-Exp) >0, (Obs-Exp)=0, and (Obs-Exp) <0, combined treatment was considered synergistic, additive, and subadditive, respectively [17]. Statistical differences between observed and expected values were defined based on p-value < 0.05.
All results were expressed as the mean±standard deviation. Statistical analysis was performed by independent sample t-test with SPSS version 17.0 software package (SPSS Inc., Chicago, USA). Statistically, p<0.05 indicated significant differences and p<0.01 represented highly significant differences between groups. A fitting curve was carried out using the linear quadratic model. The α and β parameters and inhibitory concentration 50% (IC50) were calculated using MATLAB software (MathWorks Inc., Natick, USA).
An MTT assay was performed to determine the cytotoxic effects of CAPE (1–100 µM) on MDA-MB-231 and T47D cells at 24, 48, and 72 hours (Figure 1A, B). The toxicity of CAPE was time- and concentration-dependent in the two cell lines. As shown in Figure 1A, the viability of MDA-MB-231 cells markedly decreased at high concentrations of CAPE (20–100 µM) at three different times. The cytotoxicity of CAPE on T47D cells gradually declined over 24 to 72 hours (Figure 1B). However, a remarkable reduction in the number of viable T47D cells was observed at 72 hours. The IC50 values for MDA-MB-231 and T47D cells were found to be 42.84 and 35.04 µM at 72 hours, respectively.
Figure 2 shows the effect of treating breast cancer cells with various concentrations (1–10 µM) of CAPE for 72 hours on the colony-forming ability of cells. CAPE slightly decreased the proliferation of MDA-MB-231 cells at 1 µM (p=0.023). A considerable reduction in colony number was observed at 5 and 10 µM CAPE (p=0.0001 and p=0.0002, respectively) (Figure 2A). Similar to the results for MDA-MB-231 cells, CAPE decreased the colony formation ability of T47D cells at 5 and 10 µM (p=0.0002 and p=0.0001, respectively) (Figure 2B). Images of the colonies are shown in Figure 2C and D .
Based on the results presented in the Figure 2, MDA-MB-321 and T47D cells were treated with 1 µM CAPE for 72 hours and irradiated with various doses of ionizing radiation. Figure 3 shows the cell survival curve of MDA-MB-231 and T47D cells for combined CAPE and radiation treatment. The α and β parameters calculated from the survival curve are shown in Table 1. The survival of MDA-MB-231 cells decreased at 6 Gy (p=0.0021) and 8 Gy (p=0.0004) after combination treatment in comparison to that observed after irradiation only (Figure 3A). In contrast, CAPE reduced the survival of T47D cells at 2 Gy (p=0.00002) and 4 Gy (p=0.004) (Figure 3B).
In the comet assay, DNA damage was quantified by measuring the %DNA in the tail (Figure 4A, B) and Olive tail moment (Figure 4C, D) in four different groups, including controls, CAPE alone, radiation alone, and combination of CAPE and radiation at various times after irradiation. Treatment with 1 µM CAPE alone did not change the level of DNA damage as compared with the control in the two cell lines (p>0.05). Radiation alone caused DNA damage in MDA-MB-231 and T47D cells immediately after exposure. In irradiated MDA-MB-231 cells, most damage was rapidly repaired in approximately 30 minutes after exposure. Combined treatment of cells with CAPE and radiation significantly delayed the repair process for up to 60 minutes after exposure to radiation (p=0.002). The level of DNA damage following combinational treatment was the same as the control value at 120 minutes after exposure (p=0.893). Notably, CAPE impaired DNA repair for up to 120 minutes in T47D cells. A significant difference was observed in the %DNA in the tail and Olive tail moment for 60 (p=0.023 and p=0.035) and 120 minutes (p=0.026 and p=0.016), respectively. The synergistic effect of CAPE and radiation is shown in Table 2. Changes in the amount of radiation-induced damage is depicted in Figure 5A, B for the indicated times postexposure.
The resistance to radiotherapy is a common problem leading to therapeutic failure during breast cancer treatment. Because of its resistant to radiotherapy and probability of recurrence, concerns regarding breast cancer treatment have increased [18]. Numerous studies have used a combination of radiation and chemical agents, particularly low-toxicity agents [19]. Previous studies showed that CAPE acts as a radiation sensitizer in some types of cancer. Since CAPE targets the most important radioresistance signaling pathway, it may improve the efficiency of the radiation response [8202122].
One of the most important classification factors in breast cancer cells is the presence estrogen receptors [18]. In breast cancer, estrogen receptor signaling plays a critical role in cell proliferation and survival [23]. MDA-MB-231 is the ideal cell line for triple-negative breast cancer since it lacks estrogen receptors α with minimal expression of estrogen receptor β. T47D is an estrogen receptor-positive cell line that expresses both estrogen receptors α and β [2425]. The estrogenic effect of CAPE is unclear, but its ability to bind estrogen receptors has been demonstrated previously. CAPE is a selective estrogen receptor modulator and has greater affinity to estrogen receptor β than to estrogen receptor α [23]. Therefore, estrogen receptor-positive breast cancer cells may be more susceptible to estrogen-related compounds [26].
In the present study, we evaluated the effects of combination of CAPE and radiation on ER-positive (T47D) and ER-negative (MDA-MB-231) cell lines. Based on our results, increasing concentrations of CAPE reduced the viability and colony-forming ability of MDA-MB-231 and T47D cells. CAPE exhibited anticancer effects by inhibiting cell growth and decreasing viability in a time- and dose-dependent manner [821]. As shown previously, CAPE reduced the colony formation ability of PC-3 prostate cancer cell line at 10 to 20 µM [27]. In an in vivo study, Wu et al. [28] reported that CAPE decreased the volume of tumors of MDA-MB-231 xenografts, but lower doses of CAPE were more effective in inhibiting the growth of this metastatic subgroup of breast cancer.
Our data revealed that the surviving fraction significantly decreased in cells treated with CAPE and radiation compared to that in cells subjected only to irradiation. This indicates that the radiosensitization of CAPE is associated with increasing β parameter values in MDA-MB-231 cells. In contrast, the increase in the radiosensitizing effect in T47D cells by CAPE may have been related to the greater damage at lower doses of radiation, which then acts as an α-type sensitizer. Based on a previous study, an increase in the α parameter was related to the DNA damage caused by a single hit effect of radiation interaction. This damage included double-strand breaks, which can be lethal. The changes in the β parameter are caused by two radiation interactions [29]. Thus, T47D cells are more susceptible than MDA-MB-231 cells to damage by combinational treatment with CAPE.
The capacity of cells to conduct DNA strand-break repair may be one mechanism of radiosensitivity [19]. In the comet assay, the amount of DNA damage rapidly decreased in irradiated cells. It appeared that CAPE could maintain DNA damage during combined treatment with radiation compared to in irradiated cells. Our data supported that CAPE delayed the repair mechanism by up to 120 minutes in T47D cells, but could impair DNA repair by up to 60 minutes after radiation in MDA-MB-231 cells. In the MDA-MB-231 and T47D cell lines, we observed an additive and synergistic interaction following combinational treatment. Targeting of DNA repair mechanisms and increasing radiation sensitivity using other polyphenols was described previously [14]. Radiation sensitivity may also be achieved by inhibiting the NF-κB pathway. NF-κB activation is involved in the induction of DNA repair and delay programmed cell death [12]. It was also demonstrated that CAPE inhibited the binding of NF-κB to DNA [1130]. Thus, blocking of the NF-κB pathway by CAPE prevents DNA repair.
In conclusion, our results demonstrated that CAPE acts as a radiosensitizer in breast cancer cells. CAPE inhibited clonogenicity and maintained radiation-induced DNA damage in the two cell lines, with marked effects in T47D cells. Given the similarity in structures between CAPE and estrogen, CAPE may be more effective in T47D (estrogen receptor-positive) cells than MDA-MB-231 (estrogen receptor-negative) cells. In accordance with the results of the comet assay, there is a synergistic interaction between CAPE and radiation. Further studies are needed to detect the molecular mechanism of the repair process influenced by CAPE.
Figures and Tables
Figure 1
Effect of CAPE on the viability of breast cancer cell lines. The cells were treated with 1–100 µM CAPE for 24, 48, and 72 hours and cell viability was evaluated using the MTT assay. (A) MDA-MB-231 cells. (B) T47D cells. CAPE reduced the cell viability in time- and dose-dependent manners. The data represent the mean±SD of three independent experiments.
DMSO=dimethyl sulfoxide; CAPE=caffeic acid phenethyl ester; MTT=3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide.

Figure 2
Clonogenic ability of MDA-MB-231 and T47D cells in the presence of different concentrations of CAPE. The cells were treated with 1, 5, and 10 µM CAPE for 72 hours, replated, and incubated for the indicated number of days to form colonies. (A) MDA-MB-231 cells. (B) T47D cells. (C) Representative colony formation assay of MDA-MB-231 (up) and T47D (down) cells at different concentrations of CAPE. (D) Images of single colonies of MDA-MB-231 (up) and T47D (down) cells after crystal violet staining, acquired using an inverted microscope (×5). Data represent the mean±standard deviation of two independent experiments.
DMSO=dimethyl sulfoxide; CAPE=caffeic acid phenethyl ester. *p<0.05; †p<0.001.
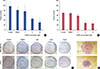
Figure 3
CAPE effects on breast cancer cell line radiosensitivity. (A) Survival curve of MDA-MB-231. (B) Survival curve of T47D cells. A colony-forming assay was performed using control cells and cells treated with 1 µM CAPE for 72 hours after exposure to radiation (2, 4, 6, and 8 Gy). The surviving fraction of group treated with CAPE plus radiation was compared to that of the irradiated group.
IR=irradiation; CAPE=caffeic acid phenethyl ester. *p<0.05; †p<0.001 represent statistical significance.

Figure 4
Level of radiation induced breaks in the presence of CAPE. MDA-MB-231 and T47D cells were treated with 1 µM CAPE for 72 hours and then exposed to radiation (6 Gy); the amount of DNA damage was determined by the comet assay immediately after irradiation until 2 hours. (A) %DNA in tail in MDA-MB-231. (B) %DNA in tail in T47D. (C) Olive tail moment in MDA-MB-231. (D) Olive tail moment in T47D. The mean±SD value of each parameter was measured from two independent experiments.
CAPE=caffeic acid phenethyl ester; IR=irradiation. *p<0.05.

Figure 5
Representative comet assay images of MDA-MB-231 and T47D cells show changes in DNA damage levels. CAPE pretreated with 1 µM CAPE for 72 hours and then irradiated. The amount of DNA damage was analyzed immediately after radiation exposure (6 Gy) up to 2 hours. (A) MDA-MB-231 cells. (B) T47D cells. Four different groups of cells were compared at different time points (control, CAPE treated, irradiated, and combination of radiation and CAPE) at 0, 30, 60, and 120 minutes after irradiation. Images of cells were acquired using a fluorescence microscope (×20) followed by staining with ethidium bromide.
CAPE=caffeic acid phenethyl ester; IR=irradiation.
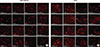
Table 1
The α and β parameter calculated from survival curve fitted to linear-quadratic model

| Treatment | MDA-MB-231 | T47D | ||
|---|---|---|---|---|
| α-value | β-value | α-value | β-value | |
| IR | 0.119 | 0.022 | 0.287 | 0.038 |
| IR+CAPE | 0.038 | 0.058 | 0.909 | 0.041 |
Table 2
Determination of synergism interaction in %DNA in tail between radiation and caffeic acid phenethyl ester

Notes
References
1. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602.

2. Hagen KR, Zeng X, Lee MY, Tucker Kahn S, Harrison Pitner MK, Zaky SS, et al. Silencing CDK4 radiosensitizes breast cancer cells by promoting apoptosis. Cell Div. 2013; 8:10.

3. Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005; 7:1630–1647.

4. Jameel JK, Rao VS, Cawkwell L, Drew PJ. Radioresistance in carcinoma of the breast. Breast. 2004; 13:452–460.

5. Nambiar D, Rajamani P, Singh RP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011; 728:139–157.

6. Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther. 2011; 130:310–324.

7. Watanabe MA, Amarante MK, Conti BJ, Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 2011; 63:1378–1386.

8. Chen MF, Wu CT, Chen YJ, Keng PC, Chen WC. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiat Res. 2004; 45:253–260.

9. Ozturk G, Ginis Z, Akyol S, Erden G, Gurel A, Akyol O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci. 2012; 16:2064–2068.
10. Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013; 65:515–526.

11. Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996; 93:9090–9095.

12. Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010; 1805:167–180.

13. Ding J, Miao ZH, Meng LH, Geng MY. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci. 2006; 27:338–344.

14. Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008; 14:1266–1273.

15. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006; 1:2315–2319.

16. Rouhani M, Goliaei B, Khodagholi F, Nikoofar A. Lithium increases radiosensitivity by abrogating DNA repair in breast cancer spheroid culture. Arch Iran Med. 2014; 17:352–360.
17. Simeone AM, Broemeling LD, Rosenblum J, Tari AM. HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogene. 2003; 22:6739–6747.

18. Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010; 32:35–48.

19. Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol. 2010; 80:1904–1914.

20. Chen YJ, Liao HF, Tsai TH, Wang SY, Shiao MS. Caffeic acid phenethyl ester preferentially sensitizes CT26 colorectal adenocarcinoma to ionizing radiation without affecting bone marrow radioresponse. Int J Radiat Oncol Biol Phys. 2005; 63:1252–1261.

21. Lin YH, Chiu JH, Tseng WS, Wong TT, Chiou SH, Yen SH. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother Pharmacol. 2006; 57:525–532.

22. Lee YY, Kao CL, Tsai PH, Tsai TH, Chiou SH, Wu WF, et al. Caffeic acid phenethyl ester preferentially enhanced radiosensitizing and increased oxidative stress in medulloblastoma cell line. Childs Nerv Syst. 2008; 24:987–994.

23. Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O'Connor OA. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J Cancer Sci Ther. 2013; 5:334–342.

24. Thomas C, Rajapaksa G, Nikolos F, Hao R, Katchy A, McCollum CW, et al. ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012; 14:R148.
25. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011; 13:215.

26. Jung BI, Kim MS, Kim HA, Kim D, Yang J, Her S, et al. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother Res. 2010; 24:295–300.

27. Lin HP, Jiang SS, Chuu CP. Caffeic acid phenethyl ester causes p21 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS One. 2012; 7:e31286.
28. Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011; 308:43–53.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download