This article has been retracted. See "Retraction note to: "Clinical Significance of the Axillary Arch in Sentinel Lymph Node Biopsy"" in Volume 18 on page 101.
Abstract
Purpose
The axillary arch is an anomalous muscle that is not infrequently encountered during axillary sentinel lymph node biopsy (SLNB) of breast cancer patients. In this study, we aimed to investigate how often the axillary arch is found during SLNB and whether it affects the intraoperative sentinel lymph node (SLN) identification rate.
Methods
We retrospectively analyzed the correlation between the presence of the axillary arch and the SLN sampling failure rate during SLNB in 1,069 patients who underwent axillary SLNB for invasive breast cancer.
Results
Of 1,069 patients who underwent SLNB, 79 patients (7.4%) had the axillary arch present. The SLNB failure rate was high when the patient's body mass index was ≥25 (p=0.026), when a single SLN mapping technique was used (p=0.012), and when the axillary arch was present (p<0.001). These three factors were also found to be statistically significant by multivariate analysis, and of these three factors, presence of the axillary arch most significantly increased the SLNB failure rate (hazard ratio, 10.96; 95% confidence interval, 4.42-27.21; p<0.001). Additionally, if the axillary arch was present, the mean operative time of SLNB was 20.8 minutes, compared to 12.5 minutes when the axillary arch was not present (p<0.001). If the axillary arch was present, the SLN was often located in a high axillary region (67%) rather than in a general low axillary location.
Sentinel lymph node biopsy (SLNB) is currently recognized as the basic surgical method for axillary nodal staging in clinically axillary node-negative breast cancer patients [1]. The most important part of SLNB is the accurate assessment of axillary nodal status.
In order to obtain accurate axillary nodal status of clinically axillary node-negative breast cancer patients, it is important to successfully identify the sentinel lymph node (SLN). Thus far, factors known to affect SLN identification rates include age, body mass index (BMI), tumor grade, SLN mapping methods, and tumor location. In particular, old age, high BMI, and use of only a single SLN mapping technique with a vital dye or radioisotope are factors that increase SLN identification failure [2,3,4]. However, according to some reports, in addition to the previously mentioned factors, the axillary arch muscle is also a factor that may reduce the SLN identification rate during SLNB [5,6,7,8].
The axillary arch is primarily known as a thin muscular anomaly extending between the latissimus dorsi muscle and pectoral muscle; however, it sometimes adheres to the coracobrachialis muscle, biceps brachi muscle, coracoid process of scapular, or axillary fascia in addition to the pectoral muscle. This anomalous axillary muscular variation is the most common muscular variation of the axillary region [9,10,11]. According to literature that has reported the axillary arch, the incidence of the axillary arch has been shown to range from 0.9% to 27% [12,13,14]. Regarding the clinical relationship between the axillary arch and the axillary SLN, Keshtgar et al. [5] reported that it may be difficult to find the SLN during axillary SLNB because in patients who have the axillary arch, the SLN is located behind the axillary arch. Also, Serpell et al. [15] reported that in patients with the axillary arch, there may be difficulties in SLNB because the axillary arch may be confused with the latissimus dorsi or pectoral muscle, which are regarded as surgical landmarks during axillary node dissection.
The purpose of this study was to investigate the frequency of the axillary arch among patients with breast cancer who undergo SLNB and to determine whether the presence of the axillary arch affects the rate of intraoperative SLN identification.
We identified 1,132 patients who were diagnosed with invasive breast cancer in the Sungkyunkwan University Samsung Medical Center from January 2012 to March 2013 and who underwent SLNB. Sixty-three patients who underwent SLNB after neoadjuvant chemotherapy were excluded; therefore, the medical records of 1,069 patients were analyzed retrospectively. These 1,069 patients underwent SLNB performed by three experienced breast surgeons. The Institutional Review Board of the Sungkyunkwan University Samsung Medical Center approved this retrospective study (approval number: 2014-07-037), and the need for informed consent was waived for the review of medical images and records.
Two mapping techniques were performed for the preoperative identification of SLNs. One technique was to inject 1 mL Technetium-99m tin-colloid, a radiopharmaceutical compound, into the subareolar region before surgery and then check for images after 1 hour with lymphoscintigraphy (frontal view and lateral view); the other technique was to inject 40 mg (5 mL) indigo carmine into the periareolar area. Intraoperative identification of the SLN was conducted by measuring radioactive counts of the axillary lymph node (increased 10 times more than the background radioactive count) using a gamma probe or by detecting the blue-stained axillary lymph node. SLN metastasis was checked by examining a hematoxylin and eosin-stained frozen biopsy specimen, and if SLN metastasis was found, additional axillary lymph node dissection was performed. SLN identification failure was defined as when the surgeons could not detect SLNs during SLNB.
All axillary arches were identified during SLNB. If there was a suspected axillary arch during preoperative computed tomography, the presence of the axillary arch was carefully investigated during SLNB (Figure 1A). In addition, we also counted the number of patients in which the axillary arch was accidewntally found during SLNB (Figure 1B). Consequently, we found the axillary arch in 79 of 1,069 patients (7.4%).
The chi-square test was used to analyze the relationship between factors and the intraoperative SLN identification rate. If there were statistically significant factors affecting the SLN identification rate in the chi-square test, multivariate analyses were conducted using the logistic regression model. An independent t-test was used to compare the mean SLNB time between the group with the axillary arch and the group without the axillary arch and also to compare the difference in the mean number of SLNs between the group with the axillary arch and the group without the axillary arch. All statistical analyses were performed using statistical software (SPSS version 20; IBM Corp., Armonk, USA). Statistical significance was established with a p-value of less than 0.05.
The median age of all patients was 49 years (range, 23-85 years), and the mean BMI was 23.2 kg/m2. Breast-conserving surgery was performed in 793 patients (74.2%), and mastectomies were performed in 276 patients (25.8%). Eight hundred and thirty-one patients (77.7%) were confirmed to have a single lesion, and invasive ductal cancer accounted for 81.6% of cases. The axillary arch muscle was found in 79 patients (7.4%) (Table 1).
Regarding SLN mapping techniques, only radioisotope was used in 335 cases (31.3%), and only vital dye was used in 235 cases (22.0%); both methods were used in 499 cases (46.7%). The median number of SLNs resected during SLNB was 3 (range, 1-9); cancer metastasis in the SLN was found in 253 cases (23.7%), and in 24 cases (2.2%), identification of the sentinel node during the SLNB was unsuccessful (Table 1).
SLN identification failure during SLNB occurred in 24 cases. Factors related to SLN identification failure were analyzed; BMI ≥25 kg/m2 (p=0.026) and presence of the axillary arch (p<0.001) were found to be significantly associated with SLNB failure rate. SLNB was found likely to be successful when conducting SLN mapping using both dye and isotope (Table 2). As shown in Table 3, multivariate analysis was performed for various factors using the logistic regression model, and patients with a BMI ≥25 kg/m2 had a 3.12-fold higher SLNB failure rate than those with a BMI <25 kg/m2. If there was an axillary arch, the SLNB was 10.96 times more likely to fail, and if only one mapping technique was used, the SLNB was about 4 to 6 times more likely to fail. The possibility of SLNB failure when there was an axillary arch present appeared to be about 10 times higher than when there was no axillary arch present, indicating that is the axillary arch is the most important factor affecting the failure rate of SLNB (hazard ratio, 10.96; 95% confidence interval, 4.42-27.21; p<0.001).
Table 4 shows the difference in SLNB results between the group with the axillary arch and the group without the axillary arch; the group with the axillary arch showed a 12.7% (10/79) SLNB failure rate, while the group without the axillary arch showed a 1.4% (14/990) failure rate. Patients with the axillary arch showed a high SLNB failure rate compared to patients without the axillary arch; thus, it was found that presence of the axillary arch makes SLNB more difficult (p<0.001). In addition, looking at the number of SLNs resected, an average of 2.4 SLNs were resected in patients without the axillary arch, in contrast to an average of 1.7 SLNs in patients with the axillary arch, indicating that statistically significant fewer nodes were obtained in those with the axillary arch (p=0.002). Also, the mean operative time of SLNB was 20.8 minutes in the group with the axillary arch versus 12.5 minutes in the group without the axillary arch; the operative time for patients with the axillary arch was an average of 8 minutes longer than that for patients without the axillary arch (p<0.001).
Of the 79 patients in whom the axillary arch was identified, a total of 69 patients, excluding 10 patients failing the SLN sampling, were classified into three groups depending on the location of the SLN in relation to the axillary arch. First, those with the SLN located behind the axillary arch or higher were classified into the high axillary group; second, those with the SLN located lower than the axillary arch were classified as the low axillary group; and finally, those with the SLN found both at a high and a low axillary location were classified as the combined group (Figure 2). The SLN was most frequently found in a high axillary location, with 46 out of 69 (67%) patients in the high axillary group (Figure 2); the low axillary and the combined group accounted for 13 patients (19%) and 10 patients (14%), respectively. The important point with these results is that if the 10 patients in the combined group (14%) were included in the high axillary group, the probability that the SLN is located behind the axillary arch or higher accounts for 81% of cases with the axillary arch.
In this study, we investigated factors affecting intraoperative SLN detection. The results showed that the intraoperative SLN sampling failure rate appeared higher when BMI was ≥25 and a single SLN mapping technique was used. These results were similar to those of papers published in the past, although age, size and histologic grade of the tumor, and factors such as multifocality proved not to affect the intraoperative SLN identification rate. In addition, this study also showed that the anomalous muscle of the axilla, the axillary arch, is a significant factor increasing the failure rates of SLNB. The axillary arch was also verified to affect the intraoperative SLN identification rate in the logistic regression model (hazard ratio, 10.96; 95% confidence interval, 4.42-27.21; p<0.001).
The axillary arch is a thin muscular anomaly extending mainly between the latissimus dorsi and pectoral muscle and is the most common muscular variation of the axillary region [16]. Several clinical studies have reported various incidence rates of the axillary arch, ranging from 0.9% to 7.48% [12,17]. The incidence of the axillary arch identified in several cadaveric dissection studies was 3% to 27%, a higher frequency compared to the incidence reported in clinical studies [12,13,14]. According to the results of our study, the axillary arch was present in 79 out of 1,069 patients, resulting in an incidence rate of 7.4%. Compared to other existing clinical studies, this incidence is similar or greater.
The results of this study showed that the intraoperative SLNB failure rate of patients with the axillary arch was 12.7% (10/79), increased by about 11% compared to the detection failure rate in patients without the axillary arch (1.4%; 14/990). It was recently reported that the presence of the axillary arch may interfere with SLN detection during SLNB; Ando et al. [6] conducted preoperative axillary multidetector row computed tomography on a total of 550 breast cancer patients, and the axillary arch was found in 59 patients. The axillary arches were divided into five types, and then the SLN identification failure rate for each type of axillary arch was assessed. The results showed that the SLN identification failure rate was 23.1% for type 2 or 3 axillary arch cases, a much higher rate compared to the failure rate of 2.2% in cases without the axillary arch [6].
We identified that the SLN is located behind the axillary arch or in the high axillar region between the axillary arch and the axillary neurovascular bundle in 67% (46/69) of patients with the axillary arch. An magnetic resonance imaging study on axillary arches reported by Guy et al. [18] showed that the axillary lymph node was found at the axillary arch level or higher in 92% (65/71) of patients with the axillary arch.
In our study, combining the rate for the high axillar region (67%) and the rate for the combined region (14%), in which the SLN is present in both the low axillary and high axillary areas, resulted in the SLN being located in the axillary arch level or higher in 81% (56/69) of cases. Therefore, most SLNs were found in the high axillary region. Several papers that have been published with respect to the general location of SLNs have reported that more than 90% of SLNs are found in the low axillary area, 1 to 2 cm lower than the axillar hairline [18,19]. However, if the axillary arch is present, as identified in our study, the SLNs are mainly located in the high axillary region.
Such an unusually high axillary location of the SLNs is hypothesized to result in difficulties in intraoperative SLN detection and to increase the SLNB failure rate. Presence of the axillary arch may cause the surgeon to confuse it with surrounding anatomic landmark muscles, such as the latissimus dorsi or pectoral muscles, thereby resulting in more surgical time spent detecting the SLNs. In our study, in the case of patients with the axillary arch, the average time for SLNB was 20.8 minutes, about 8 minutes more than the operation time of 12.5 minutes in patients without the axillary arch. The average number of SLNs removed in cases with the axillary arch and in those without the axillary arch was 1.7 and 2.4, respectively; if the axillary arch was present, the number of identified SLNs was small. This is because it is difficult to understand the anatomical structure of the axillar area during surgery due to the presence of the axillary arch and the abnormal locations of the SLNs.
In conclusion, it was revealed that a BMI of ≥25 kg/m2, use of a single SLN mapping technique, and the presence of the axillary arch are factors that affect the intraoperative SLN identification rate. Among these factors, the axillary arch is the most significant factor causing SLN detection failure. As can be seen in this study, SLNs in the patients with axillary arch are mainly located in the high axillary region. Therefore, if the axillary arch is found during SLNB, it is important to check whether an SLN is located behind the axillary arch or in the high axillar region above the axillary arch to reduce the SLNB failure rate.
Figures and Tables
Figure 1
The relationship between axillary arch and the location of sentinel lymph nodes (SLNs). (A) The findings of chest computed tomography for the location of SLN in a 58-year-old breast cancer patient with axillary arch; the SLN (white arrow) can be seen located in the top of the axillary arch (yellow arrows). (B) The SLN (thick arrow) can be seen located above the axillary arch, and the lymphatic flow (thin arrows) colored with dye is found to flow into the SLN at the top of the axillary arch.
PM=pectoralis muscle; LD=latissimus dorsi; AA=axillary arch.

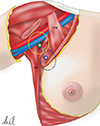
Figure 2
The illustration for the relationship between the axillary arch and the location of sentinel lymph nodes (SLNs). The yellow-dotted circle shown in the back and top of the axillary arch is the high axillary group of the SLN, and the blue-dotted circle is the low axillary group.
PM=pectoralis muscle; LD=latissimus dorsi; AA=axillary arch.
References
1. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997; 349:1864–1867.

2. Goyal A, Newcombe RG, Chhabra A, Mansel RE. ALMANAC Trialists Group. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer: results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006; 99:203–208.

3. Derossis AM, Fey J, Yeung H, Yeh SD, Heerdt AS, Petrek J, et al. A trend analysis of the relative value of blue dye and isotope localization in 2,000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg. 2001; 193:473–478.

4. Cox CE, Dupont E, Whitehead GF, Ebert MD, Nguyen K, Peltz ES, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J. 2002; 8:88–91.

5. Keshtgar MR, Saunders C, Ell PJ, Baum M. Langer's axillary arch in association with sentinel lymph node. Breast. 1999; 8:152–153.

6. Ando J, Kitamura T, Kuroki Y, Igarashi S. Preoperative diagnosis of the axillary arch with multidetector row computed tomography and the axillary arch in association with anatomical problems of sentinel lymph node biopsy. Breast Cancer. 2010; 17:3–8.

7. Chêne G, Le Bouëdec G, Dauplat J. Arch and sentinel: surgical technique of sentinel node biopsy with the axillopectoral muscle. Gynecol Obstet Fertil. 2007; 35:25–29.
8. Karanlik H, Fathalizadeh A, Ilhan B, Serin K, Kurul S. Axillary arch may affect axillary lymphadenectomy. Breast Care (Basel). 2013; 8:424–427.

9. Bergman RA. Doubled pectoralis quartus, axillary arch, chondroepitrochlearis, and the twist of the tendon of pectoralis major. Anat Anz. 1991; 173:23–26.
10. Del Sol M, Olave E. Elevator muscle of the tendon of latissimus dorsi muscle. Clin Anat. 2005; 18:112–114.

11. Georgiev GP, Jelev L, Surchev L. Axillary arch in Bulgarian population: clinical significance of the arches. Clin Anat. 2007; 20:286–291.

12. Ridgway PF, Collins AM, McCready DR. The surgical importance of an axillary arch in sentinel node biopsy. Surg Radiol Anat. 2011; 33:147–149.

13. Petrek JA, Blackwood MM. Axillary dissection: current practice and technique. Curr Probl Surg. 1995; 32:257–323.

14. Besana-Ciani I, Greenall MJ. Langer's axillary arch: anatomy, embryological features and surgical implications. Surgeon. 2005; 3:325–327.

15. Serpell JW, Baum M. Significance of 'Langer's axillary arch' in axillary dissection. Aust N Z J Surg. 1991; 61:310–312.

16. Daniels IR, della Rovere GQ. The axillary arch of Langer: the most common muscular variation in the axilla. Breast Cancer Res Treat. 2000; 59:77–80.

17. Natsis K, Vlasis K, Totlis T, Paraskevas G, Noussios G, Skandalakis P, et al. Abnormal muscles that may affect axillary lymphadenectomy: surgical anatomy. Breast Cancer Res Treat. 2010; 120:77–82.