Abstract
Purpose
Metastatic status of internal mammary lymph node (IMLN) has a clinical importance in assessing the stage and prognosis of breast cancer. But, when metastasis of IMLN is suspected; the management is controversial. We retrospectively reviewed 36 breast cancer patients who underwent IMLN biopsy, and investigated the pathologic status of IMLN which suspected metastasis with positron emission tomography and computed tomography (PET/CT).
Methods
From January 2007 to December 2012, 36 patients underwent IMLN biopsy for suspected IMLN metastasis on PET/CT, when diagnosed with primary or recurrent breast cancer. Clinicopathologic features of these patients and metastatic status of IMLNs were investigated.
Results
A total of 36 patients were included in this study. Twenty-four patients diagnosed with primary breast cancer and 12 patients diagnosed with recurrent breast cancer underwent IMLN biopsy. The mean number of IMLNs was 2.72±2.05, and the total metastatic rate of IMLNs was 72.2% (26 out of 36). IMLN metastasis was confirmed on pathologic examination in 19 patients (79.2%, 19 out of 24) with primary breast cancer and in 7 patients (58.3%, 7 out of 12) with recurrent breast cancer. The mean standardized uptake values of metastatic and nonmetastatic IMLNs in primary breast cancer were 3.50±2.51 and 3.72±3.55, respectively and those of metastatic and nonmetastatic IMLN in recurrent breast cancer were 3.92±2.67 and 4.12±3.57, respectively. In both groups, there was no statistically significant difference between the SUVs of metastatic and nonmetastatic IMLNs (p=0.291 and p=0.951, respectively).
Conclusion
Due to the recent advances in diagnostic and surgical skills, IMLN biopsy can be performed safely without any complications without performing radical mastectomy. If IMLN metastasis is suspected on PET/CT, IMLN biopsy is useful to assess the exact stage and to determine the treatment for breast cancer. Further follow-up studies are needed to assess the locoregional recurrence and to compare the improvement in overall survival and disease-free survival.
Like the axillary lymph node, the internal mammary lymph node (IMLN) chain is a first lymphatic drainage site in breast cancer; however, the importance of its management has long been debated. Historically, between the 1940s and 1960s, the surgery of IMLN was performed during the classical Halsted radical mastectomy with extrapleural resection of the internal mammary chain (extended radical mastectomy) [1-3]. Some studies have reported a high metastatic rate of IMLNs (44%-65%) in breast cancers with medial tumors and positive axillary nodes [4-6]. However, a multicentric randomized clinical trial, which started in 1962, did not show any survival benefit for radical dissection of IMLNs [7,8], and hence extended radical mastectomy (ERM) has since been abandoned.
Currently, the TNM staging of the 6th American Joint Committee on Cancer (AJCC) is determined by metastatic status of IMLNs and the National Comprehensive Cancer Network Clinical Practice Guidelines recommended considering radiotherapy for patients with suspected IMLN metastasis. However, for determining the direction of treatment and prognosis for these patients, an accurate assessment of IMLN metastasis is the most important consideration. The presence of metastatic IMLNs can change the tumor stage and can determine the direction of treatment.
We conducted a retrospective review of 36 breast cancer patients who underwent IMLN biopsy for suspected IMLN metastasis on positron emission tomography and computed tomography (PET/CT) and identified the pathologic status of IMLNs. By the PET/CT-guided removal of IMLNs suspected for harboring metastasis, we investigated the diagnostic value of PET/CT for IMLN metastasis and tried to identify the exact pathologic stage of breast cancer and determine the direction of treatment.
From January 2007 to December 2012, at the Yeungnam University Hospital, 2,758 patients were diagnosed with primary breast cancer and received surgery. In this period, PET/CT was conducted in 1,978 patients and IMLN metastasis was suspected in 133 patients before the initial operation or during the follow-up period. Among these 133 patients, after excluding the patients with combined IMLN and distant metastasis, 40 patients had only IMLN metastasis and underwent IMLN biopsy based on the PET/CT findings. Fine needle aspiration cytology or core needle biopsy was not conducted before IMLN biopsy. Excluding the 4 patients who had incomplete data, a total of 36 patients were included in this study. Clinicopathologic features of these patients and the pathological metastatic status of IMLNs were retrospectively investigated. This study was approved by the Institutional Review Board of Yeungnam University College of Medicine (IRB No. YUH-13-0369-B4).
All patients with primary breast cancer underwent preoperative lymphoscintigraphy, ultrasound, and PET/CT. Patients with locoregionally recurrent breast cancer underwent ultrasound and PET/CT. IMLN biopsy was performed in 36 patients with suspicion of metastasis on PET/CT. On PET/CT, we defined suspected metastatic IMLNs as those with an uptake clearly greater than the adjacent background in the first-fifth intercostal space along the lateral sternal border. For localization of the IMLNs that were identified on PET/CT, we checked the level of the intercostal space through physical examination and radiologic findings.
After administration of general anesthesia, surgery was performed through a skin incision measuring approximately 3 to 4 cm over the location of the suspected IMLNs. If radical mastectomy (RM) or modified radical mastectomy (MRM) was performed simultaneously, the surgery was performed through the skin incision for mastectomy. For breast conserving surgery (BCS), we used either the same skin incision or a separate skin incision.
We dissected the pectoralis major muscle and cut the intercostal muscle. We then found the IMLNs in the fatty tissue along the internal mammary vessels on the surface of the parietal pleura (Figure 1). In addition to the initially approached intercostal space, we performed a lymph node biopsy at the upper or lower level of the intercostal space.
Resected IMLN >5 mm in size were sectioned at 5 mm intervals along the long axis, and the nodes <5 mm in size were sectioned at their largest diameter. Routine hematoxylin and eosin staining was performed. The diagnostic criterion for lymph node metastasis, according to the 6th AJCC, was a cluster of malignant cells >0.2 mm in the lymph node.
A total of 36 patients were included in this study. Twenty-four patients with primary breast cancer and 12 patients with locoregionally recurrent breast cancer underwent IMLN biopsy. In patients with primary breast cancer, the mean tumor size was 3.55±1.81 cm and the mean number of metastatic axillary lymph nodes was 8.58±7.30. In patients with recurrent breast cancer, the primary tumor size was 2.87±2.07 cm. IMLN biopsy was performed through a separate incision (in 14 patients) or through the same incision as that in RM (in 2 patients), MRM (in 17 patients), nipple areola skin-sparing mastectomy (in 1 patient), and BCS (in 2 patients) (Table 1). Rib resection was not performed in any of the cases.
All patients underwent a routine postoperative chest X-ray, and there were no specific complications, such as pneumothorax or hemothorax. Postoperatively, patients received chemotherapy, hormonal therapy, or radiotherapy according to the biopsy report.
The mean number of IMLNs was 2.72±2.05, and the total metastatic rate of IMLNs was 72.2% (26/36). IMLN metastasis was confirmed on pathologic examination in 19 patients (79.2%, 19/24) with primary breast cancer and in 7 patients (58.3%, 7/12) with recurrent breast cancer. The mean standardized uptake values (SUV) of metastatic and nonmetastatic IMLNs in primary breast cancer were 2.06±1.39 and 2.89±1.56, respectively and those of metastatic and nonmetastatic IMLNs in recurrent breast cancer were 4.58±3.11 and 4.43±4.63, respectively. In both groups, there was no statistically significant difference between the SUVs of metastatic and nonmetastatic IMLNs (p=0.291 and p=0.951). The patients with pathologically confirmed metastatic IMLNs showed a tendency to have a larger primary tumor size and a larger number of metastatic axillary lymph nodes. However, there was no statistically significant difference between both groups (Tables 2, 3).
We performed additional lymph node biopsy at the upper or lower level of the intercostal space in 17 patients. When IMLN metastasis of originally suspicious intercostal space was pathologically confirmed, pathological metastatic rate of IMLN in upper and lower intercostal space was 66.7% (6/9). Only IMLN metastasis without axillary node metastasis was found in 4 patients (11.1%, 4/36), and the tumor location in these patients was the inner quadrant or the central quadrant (Table 4).
Assessment of the axillary lymph nodes has been accepted as a part of the standard operative procedure in breast cancer surgery, and axillary dissection and radiotherapy of the axilla result in excellent locoregional control of breast cancer. However, the management of IMLNs has always been debatable. Approximately 3% to 21.8% of lymph from the breast is estimated to flow into the internal mammary chain [9,10] and tumors with medial location, axillary lymph node metastasis, and large size have a higher rate (44%-65%) of IMLN metastasis [2,3,11]. However, in 1985, Veronesi et al. [2] conducted an analysis of 1,119 patients who underwent ERM and reported a relatively similar prognosis between the patients with only axillary metastasis and those with only IMLN metastasis (10 year survival rate, 47.2% vs. 51.9%).
One of the most important reasons for controversy regarding the management of IMLNs is the lack of survival benefits. In 1954, through an analysis of ERM, Handley and Thackray [12] first described the detailed surgical technique of IMLN biopsy and reported a metastatic rate of IMLNs of 33%. However, a multinational randomized trial conducted in 1963 showed that there is no statistically significant survival benefit from ERM compared with RM, and various complications such as chest wall deformity, pneumothorax, and hemothorax have been reported during and after ERM [7,8,13-15]. ERM has since been abandoned. However, at this point in time, with advanced diagnostic and treatment techniques, we should reinterpret these previous results. Actually, in the study by Veronesi et al. [1], they did not administer adjuvant therapy, such as chemotherapy, hormonal therapy, or radiotherapy. Currently, there has been a marked advancement in the preoperative diagnostic techniques. Ultrasonography, mammography, magnetic resonance imaging, PET/CT, and CT. are performed as preoperative examinations, instead of only being used CT as preoperative diagnostic method in the past. These diagnostic methods provide more information on precise localization of the tumor or distant metastasis. The use of concurrent local and systemic treatments for breast cancer is currently being emphasized. MRM or BCS is preferred over RM as a local treatment; and adjuvant therapies, including chemotherapy, hormonal therapy, and target therapy are administered as a systemic treatment after local treatment.
By the PET/CT-guided biopsy of IMLNs suspected for harboring metastasis, we investigated the diagnostic value of PET/CT for IMLN metastasis and tried to identify the exact pathologic stage of breast cancer and determine the direction of treatment. In the past, ERM was performed based on RM with an additional resection of ribs and removal of lymph nodes and fat over the parietal pleura [3]. In our study, the IMLNs were removed from the site suspicious for metastasis on diagnostic methods without performing rib resection. We could avoid serious complications such as pneumothorax and hemothorax. To date, there is no study of IMLN biopsy when IMLN metastasis was suspected on PET/CT. Although many studies have reported on the accuracy of PET/CT in evaluating the status of IMLNs, there was no pathological confirmation of metastasis. Most of the studies only assessed the change in the SUVs on the follow-up PET/CT, and no long-term follow-up results of PET/CT have been reported [16-18]. According to the study by Eubank et al. [17] in 2001, sensitivity and specificity of PET/CT was reported to be 85% and 90%, respectively; however, in our study, the metastatic rate of pathologically confirmed IMLNs detected on PET/CT was 72.2% (26/36).
Although breast cancer patients with only IMLN recurrence are rare, metastasis to IMLNs raises the possibility of distant metastasis and is associated with a decreased survival rate [16,19,20]. The current National Comprehensive Cancer Network Clinical Practice guidelines recommend the use of radiotherapy when IMLN metastasis is suspected clinically. The Early Breast Cancer Trialists' Collaborative Group, in 2005, reported on the effect of locoregional control on long-term survival and the importance of radiotherapy [21]. Although many large retrospective studies have reported on the additional benefits of radiotherapy, most studies have patient and treatment selection biases, which make it difficult to interpret the results [22-26]. Through radiotherapy, patients can expect to achieve an excellent local control. However, ribs and sternum may interrupt the radiation in patients with metastatic IMLNs, which may cause a decrease in the treatment effect. Also, radiation can cause radiation pneumonitis and cardiac toxicity, such as ischemic heart disease, in patients with actually nonmetastatic IMLNs [21,27]. In particular, cardiac toxicity is increased by the additional use of systemic therapy such as anthracycline or trastuzumab. There are no published results of clinical trials assessing the effect of radiotherapy on IMLNs. We dissected the IMLNs when metastasis was suspected on PET/CT. When IMLN metastasis was confirmed pathologically, the patients received additional radiotherapy. In case of pathologically confirmed nonmetastatic IMLNs, unnecessary radiation could be avoided in the patients.
In our study, we attempted to confirm IMLN metastasis pathologically and to improve the survival rate through simple surgical removal and additional radiotherapy.
However, our study has several limitations. The first question is whether it is possible to remove all the metastatic IMLNs. Although we removed most of the lymph nodes and fat over the intercostal space that were suspected of harboring metastasis on PET/CT, and in some cases, we also checked the upper or lower levels of the intercostal space, some lymph nodes may have remained behind the sternum and ribs. However, pathological confirmation of metastasis can provide directions in making decisions regarding further treatment such as additional radiotherapy and adjuvant therapy after surgical removal, which can have a synergistic effect. The second question pertains to the long-term results. In our studies, most patients with suspected metastatic IMLNs on PET/CT had locally advanced breast cancer such as pathological stage IIIA-C. The question whether the removal of IMLNs has an effect on the prognosis of these patients can be addressed by performing additional studies.
According to Veronesi et al. [2] in 1985, 9.1% (51/563) of patients who underwent ERM had only IMLN metastasis without axillary LN metastasis. If the patient with a large tumor or a medially located tumor did not receive an exact assessment regarding IMLN status, they could not receive appropriate treatment and would be classified into a poor prognostic group.
Due to the recent advances in diagnostic and surgical skills, IMLN biopsy without RM can be performed safely without any complications. Although, PET/CT could provide information about the clinical stage, the pathologic stage is confirmed through IMLN biopsy. If the SUV of IMLNs on PET/CT is considered, IMLN biopsy is useful for assessing locoregional control and avoiding unnecessary radiation. Further follow-up studies are needed in order to assess the locoegional recurrence and to compare the improvement in overall survival and disease-free survival.
Figures and Tables
Figure 1
Operation field showed internal mammary lymph node (IMLN) biopsy after modified radical mastectomy. (A) Pectoralis major muscle was disseted at the level of 3rd intercostal space. (B) IMLN was exposed after cutting intercostal muscle. (C) After removing IMLN, internal mammary vessels were left on the surface of the pairietal pleura.
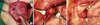
Table 2
Comparison between pathologic nonmetastatic and metastatic internal mammary lymph node in primary breast cancer (n=24)

Table 3
Comparison between pathologic nonmetastatic and metastatic internal mammary lymph node in recurrent breast cancer (n=12)

References
1. Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients: 30-year results of a randomised trial. Eur J Cancer. 1999; 35:1320–1325.

2. Veronesi U, Cascinelli N, Greco M, Bufalino R, Morabito A, Galluzzo D, et al. Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg. 1985; 202:702–707.

3. Livingston SF, Arlen M. The extended extrapleural radical mastectomy: its role in the treatment of carcinoma of the breast. Ann Surg. 1974; 179:260–265.
4. Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer. 1977; 39:533–538.

5. Caceres E. Incidence of metastasis in the internal mammary chain in operable carcinoma of the breast and 5 year results. Acta Unio Int Contra Cancrum. 1963; 19:1566–1569.
6. Urban JA, Marjani MA. Significance of internal mammary lymph node metastases in breast cancer. Am J Roentgenol Radium Ther Nucl Med. 1971; 111:130–136.

7. Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. 1983; 51:1941–1943.

8. Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981; 47:170–175.

9. Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004; 239:232–237.

10. Hultborn KA, Larsson LG, Ragnhult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal Au198. Acta Radiol. 1955; 43:52–64.

11. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387.

12. Handley RS, Thackray AC. Invasion of internal mammary lymph nodes in carcinoma of the breast. Br Med J. 1954; 1:61–63.

13. Lacour J, Lê MG, Hill C, Kramar A, Contesso G, Sarrazin D. Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol. 1987; 13:309–314.
14. Meier P, Ferguson DJ, Karrison T. A controlled trial of extended radical versus radical mastectomy. Ten-year results. Cancer. 1989; 63:188–195.

15. Morimoto T, Monden Y, Takashima S, Itoh S, Kimura T, Yamamoto H, et al. Five-year results of a randomized clinical trial comparing modified radical mastectomy and extended radical mastectomy for stage II breast cancer. Surg Today. 1994; 24:210–214.

16. Bellon JR, Livingston RB, Eubank WB, Gralow JR, Ellis GK, Dunnwald LK, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004; 27:407–410.

17. Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001; 19:3516–3523.

18. Jones A, Bernstein V, Davis N, Bryce C, Wilson D, Mankoff D. Pilot feasibility study to assess the utility of PET scanning in the pre-operative evaluation of internal mammary nodes in breast cancer patients presenting with medial hemisphere tumors. Clin Positron Imaging. 1999; 2:331.

19. Halverson KJ, Taylor ME, Perez CA, Garcia DM, Myerson R, Philpott G, et al. Regional nodal management and patterns of failure following conservative surgery and radiation therapy for stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 1993; 26:593–599.

20. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18:2817–2827.

21. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–2106.

22. Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management: a systematic review. J Clin Oncol. 2008; 26:4981–4989.

23. Fowble B, Hanlon A, Freedman G, Nicolaou N, Hoffman J, Sigurdson E, et al. Internal mammary node irradiation neither decreases distant metastases nor improves survival in stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 2000; 47:883–894.

24. Lê MG, Arriagada R, de Vathaire F, Dewar J, Fontaine F, Lacour J, et al. Can internal mammary chain treatment decrease the risk of death for patients with medial breast cancers and positive axillary lymph nodes? Cancer. 1990; 66:2313–2318.

25. Obedian E, Haffty BG. Internal mammary nodal irradiation in conservatively-managed breast cancer patients: is there a benefit? Int J Radiat Oncol Biol Phys. 1999; 44:997–1003.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download