Abstract
Purpose
As doxorubicin cardiotoxicity is considered irreversible, early detection of cardiotoxicity and prevention of overt heart failure is essential. Although there are monitoring guidelines for cardiotoxicity, optimal timing for early detection of subclinical doxorubicin cardiotoxicity is still obscure. The purpose of this study is to determine optimal timing of cardiac monitoring and risk factors for early detection of doxorubicin cardiotoxicity in young adult patients with breast cancer.
Methods
Medical records of 1,013 breast cancer patients diagnosed from January 2009 to December 2010 is being reviewed and analyzed. Properly monitored patients are defined as patients who underwent transthoracic echocardiography before and after the chemotherapy. The definition of subclinical cardiotoxicity (SC) either decreases left ventricular ejection fraction (LVEF) more than 10% or the LVEF declines under 55% from baseline without heart failure symptoms.
Results
Twenty-nine out of 174 (16.7%) properly monitored young adult female patients (mean age, 52±10 years old) developed SC. The mean interval of cardiac evaluation of SC group was 5.5±3.0 months. Among the risk factors, the history of coronary artery disease, cumulative dose of doxorubicin ≥300 mg/m2 and use of trastuzumab after doxorubicin therapy were associated with development of SC. At cumulative dose of doxorubicin 244.5 mg/m2, SC can be predicted (sensitivity, 71.4%; specificity, 70.9%; area under the curve, 0.741; 95% confidence interval, 0.608-0.874; p=0.001).
Cardiotoxicity associated with doxorubicin and trastuzumab is a well known obstacle in the treatment of breast cancer patients. The mechanisms of doxorubicin cardiotoxicity are necrosis and apoptosis of cardiac myocyte and myocardial fibrosis [1-3], and as a result, doxorubicin cardiotoxicity is considered to be irreversible [1-3], so the early detection of left ventricular (LV) dysfunction and prevention of overt heart failure is important. There are number of trials that have suggested that early decline of LV ejection fraction (LVEF) after doxorubicin chemotherapy is associated with long term development of cardiac dysfunction [4-6]. The early detection of cardiotoxicity and proper management, even in asymptomatic patients, is considered critical [7]. Guidelines for cardiac function monitoring of doxorubicin cardiotoxicity have been recommended [8,9], but these guidelines are not strictly followed in clinical practice [7,10,11] and new methods for early detection of anthracycline cardiotoxicity have been suggested [12-18]. In patients aging <15 years or >60 years, cardiac function monitoring after administration of relatively low cumulative dose of doxorubicin is recommended [19]. However, in patients aging between 15 years to 60 years, cardiac function monitoring is recommended after administration of cumulative dose of doxorubicin 300 mg/m2 [19]. The objective of this study was to determine whether the early cardiac function monitoring in middle-aged breast cancer patients at relatively low cumulative dose of doxorubicin is effective for detecting subclinical doxorubicin cardiotoxicity (SC).
A review of medical records identified a total of 1,013 breast cancer patients who received doxorubicin-containing chemotherapeutic regimen at three hospitals in The Catholic University of Korea (Seoul St. Mary's Hospital, Yeouido St. Mary's Hospital, Bucheon St. Mary's Hospital) from January 1, 2009, through December 31, 2010. Patients with ages ranging from 18 years to 65 years who underwent the cardiac function monitoring with two-dimensional transthoracic echocardiography (TTE) were selected for this study. All TTE images were reviewed and interpreted by experts. LVEF was measured by modified Simpson's method with the use of TTE modality from thee hospitals, GE vivid 7, GE vivid E-9 (GE Healthcare, Fairfield, USA), Philips IE 33 (Philips Andover, Andover, USA) and interobserver and intraobserver variability was less than 10%, respectively. Patients who had symptoms and signs of congestive heart failure or baseline LVEF <55%, were excluded. A total of 496 young adult patients with breast cancer underwent doxorubicin administration and cardiac monitoring by TTE. Of the 496 patients, those who had baseline TTE performed before chemotherapy and at least one follow-up TTE performed after administration of doxorubicin were considered to be properly monitored. Cardiotoxicity was defined when the LVEF decreases more than 10% from the baseline or the LVEF declines under 55% [7,20]. The SC was defined as the development of cardiotocixity without symptoms or signs of congestive heart failure.
Medical charts were reviewed in detail to collect clinical data including cancer characteristics, type of cancer treatment, chemotherapeutic regimens, the cumulative dosage of doxorubicin, other demographic data and cardiovascular risk factors such as hypertension, diabetes, coronary artery disease (CAD). Patients who were prescribed with medicine for the treatment of hypertension, diabetes, and CAD before diagnosis of breast cancer or started medication during the chemotherapeutic period were classified as having risk factors. The Institutional Review Board of The Catholic University of Korea has approved of this study (IRB number: XC10RIMI0091S).
Continuous variables are presented as mean±standard deviation and categorical data are presented as absolute value and percentage. Continuous variables are being compared by paired t-test or analysis of variance (ANOVA). Categorical data are being compared by using the chi-square test or Fisher's exact test. Odds ratios and their 95% confidence intervals calculated from logistic regression analysis are used to evaluate the relationship of risk factors to the development of SC. Receiver operating characteristic (ROC) curve analysis are applied to determine the cut-off value of the cumulative dosage of doxorubicin for prediction of SC. All analyses are performed by using SAS software version 9.1 (SAS Institute, Cary, USA).
The baseline characteristics of 174 properly monitored patients are summarized in Table 1. All patients were female and with a mean age of 52±10 years old and mean follow-up period was 16.5±4.3 months. Twenty-nine patients (16.7%) developed SC. Comparisons of baseline characteristics of the SC groups and the non-SC groups are also listed in Table 1. The baseline LVEF values from the two groups showed no significant difference. The cumulative dose of doxorubicin of SC group was 326.1±102.9 mg/m2 and was significantly higher than non-SC group (258.1±68.8 mg/m2, p=0.002). The proportion of patients receiving more than 300 mg/m2 of doxorubicin was 53.6% in SC groups was significantly higher than non-SC groups (16.4%, p<0.001). The proportion of patients with a CAD history was 24.1% which was also significantly higher than non-SC groups (3.4%, p<0.001). Fifty-seven patients (32.7%) were treated with trastuzumab after doxorubicin chemotherapy. From these 57 patients who received trastuzumab, 14 patients (22.8%) developed SC.
Figure 1 demonstrates inconsistency in cardiac function assessment during chemotherapy. The first follow-up being monitored after initial administration of doxorubicin was performed at various intervals ranging from 2 to 12 months (mean interval, 5.5±3.0 months). Patients who received trastuzumab (TZ) after doxorubicin (DOX) underwent an earlier monitoring (DOX plus TZ group) than patients who were only treated with doxorubicin (DOX group) at the timing of monitoring was before the administration of trastuzumab. Only 6 out of 57 patients in DOX plus TZ group were examined for cardiac function 6 months after the initial doxorubicin therapy.
Among the risk factors for doxorubicin cardiotoxicity, with a history of CAD, the use of trastuzumab after doxorubicin therapy and cumulative dosage of doxorubicin greater than or equal to 300 mg/m2 were associated with development of SC (Table 2). Two out of 14 patients who developed SC after trastuzumab therapy had already developed SC after doxorubicin therapy. However, those patients were treated with trastuzumab because their LVEF remained above the standard limit (>55%), even though SC had already developed before trastuzumab therapy. But eventually, their LVEF declined to less than 55%. It seemed likely that patients with left-sided breast cancer who underwent the radiation therapy are inclined to higher incidence of doxorubicin-induced cardiotoxicity than patients with right-sided breast cancer, although the differences were not statistically significant.
Comparisons of the DOX group and the DOX plus TZ group are summarized in Table 3. There was no significant difference between groups in term of cumulative dose of doxorubicin and the proportion of patients with a cumulative dosage of doxorubicin ≥300 mg/m2. Interval to first follow-up TTE of DOX plus TZ group was 4.8±2.2 months and was significantly earlier than DOX group (5.8±3.4 months, p=0.026). DOX plus TZ group underwent significantly more TTE follow-up than DOX group (4±2 times vs. 2±1 times, p<0.001). There were no significant differences between the groups in terms of baseline LVEF and LVEF after doxorubicin therapy. But LVEF values during the chemotherapeutic period decreased significantly after doxorubicin therapy (Table 4). The LVEF of the DOX plus TZ group gradually decreased after doxorubicin and then trastuzumab therapy.
The cut-off value of the cumulative dosage of doxorubicin for detecting SC is demonstrated in Figure 2. The 12 patients who developed SC after the additional trastuzumab therapy were excluded from ROC curve analysis. At cumulative dosage of doxorubicin 244.5 mg/m2, the SC can be predicted (sensitivity, 71.4%; specificity, 70.9%; area under the curve, 0.741; 95% confidence interval, 0.608-0.874; p=0.001).
Our results indicate that early cardiac function monitoring is effective for the detection of SC in young adult patients with breast cancer who are being treated with doxorubicin. Although it was previously established that doxorubicin is cardiotoxic and there are recommended guidelines [8,9] for monitoring the cardiotoxicity, only a small number of patients were monitored properly. Previous studies [7,10,11] pointed out that these recommendations [8,9] are inconsistent and are difficult to follow in clinical practice, different cumulative dosage of doxorubicin for cardiac function monitoring according to age [19] may also result in inappropriate cardiac function monitoring. Considering that the early decline of LVEF is associated with development of the subsequent cardiomyopathy [11] and the early treatment of cardiotoxicity can lead to recovery of LVEF [21], thus early detection is essential. Estimating the underlying cardiovascular risk factors, detecting temporary events, and identifying subclinical changes may be helpful in the early detection and prediction of subsequent cardiomyopathy [22].
Many studies have suggested effective methods for the early detection or prediction of anthracycline- and trastuzumab-induced cardiotoxicity. Tissue Doppler imaging [12-14], strain rate and strain imaging [13,15,16], magnetic resonance imaging [17], N-terminal pro-B-type natriuretic peptide, and troponin I [18] all play a role in early detection. But in clinical practice, precise and easy-to-follow monitoring guidelines are necessary and proper timing of cardiac function monitoring for the early detection of cardiotocity is also important. One previous study reported that decreased LVEF was frequently noted 3 weeks after completion of the fourth cycle of doxorubicin plus cyclophosphamide chemotherapy [23]. The present study produced similar results, showing that a cumulative dosage of 244.5 mg/m2 of doxorubicin was approximately same dose as four cycles of doxorubicin chemotherapy. This finding suggests that performing cardiac function evaluation when cumulative dose of doxorubicin reaches 244.5 mg/m2 is a reasonable monitoring strategy for detection of SC in young adult patients with breast cancer.
Trastuzumab is also a well-known cardiotoxic agent used for treatment of breast cancer patients. It is believed that trastuzumab may cause dysfunction and apoptosis of cardiomyocyte by disrupting epidermal growth factor signaling pathway or by inducing alterations in protein expression such as Bcl-2, Bcl-sS, and BAX [24,25]. Unlike the doxorubicin, trastuzumab cardiotoxicity is considered reversible [24]. It appears that the monitoring guidelines for trastuzumab cardiotoxicity is more precise and easy-to-follow than anthracycline [26] and encourages clinician to follow the recommendations more carefully. However, there is a study which demonstrates that guidelines are still not strictly followed by clinicians in breast cancer patients treated with trastuzumab [27]. But in this study, the DOX plus TZ group underwent earlier and more frequent cardiac function monitoring during the chemotherapeutic period than DOX group and it could be the result from the precise monitoring guidelines of trastuzumab cardiotoxicity. Table 4 shows that a combination of doxorubicin and trastuzumab results in a gradual decline of LVEF, especially when trastuzumab was administered after initial doxorubicin therapy. This result is consistent with the concept that a combination of doxorubicin and trastuzumab can cause more damage to LV function and can result in a greater decline of LVEF values. Therefore, closer observations of cardiac function are necessary when considering combination chemotherapy or when changing the drug regimen.
This study has several limitations. Although the data was being collected from three hospitals, a relatively small number of patients were analyzed. Most of patients missed the baseline TTE and were excluded from the analysis, which paradoxically emphasizes the importance of baseline cardiac function monitoring. The small sample size made it difficult to detect meaningful associations with risk factors such as hypertension, diabetes and radiation therapy to the SC development, but such risk factors may be associated with the development of overt heart failures or poor clinical outcomes. This study is insufficient to demonstrate the long-term outcome of patients with SC because all patients were undergoing chemotherapy and information about cardiac function after the completion of chemotherapy was not available for analysis. As this study is focused on the early detection of SC, the management of early detected SC is not being analyzed. It is clear that further study is needed to elucidate the effects of early detection and early proper management of SC on the clinical course of doxorubicin-induced cardiotoxicity. Other echocardiographic methods which might be more sensitive in detecting subclinical myocardial dysfunction such as myocardial performance index, tissue Doppler imaging, speckle tracking imaging and should be considered for future prospective studies. Finally, this study did not analyze the effects of other chemotherapeutic agents such as paclitaxel and cyclophosphamide, which are being used in combination with doxorubicin and could also have possible cardiotoxic effects.
This study suggests that performing early monitoring before reaching cumulative dose of doxorubicin 300 mg/m2 might be a proper strategy for early detection of SC in young adult patients with breast cancer. The SC developed at a relatively low cumulative dosage of doxorubicin in young adult patients with breast cancer was common and thus necessary to monitor cardiac function earlier. Precise monitoring guideline for trastuzumab cardiotoxicity resulted in more frequent and earlier monitoring of cardiac function during chemotherapy. Closer observations of cardiac function are necessary when trastuzumab therapy is planned after doxorubicin administration or vice versa.
Figures and Tables
Figure 1
Time period to the first follow-up of cardiac function monitoring after initial doxorubicin therapy. Varying times to first follow-up transthoracic echocardiography after the initial dose of doxorubicin demonstrates the lack of uniformity in cardiac function monitoring during chemotherapy.
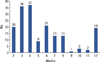
Figure 2
Receiver operating characteristics curve to determine the cumulative dosage of doxorubicin that predicts subclinical cardiotoxicity. Area under the curve and 95% confidence interval are 0.741 (0.608-0.874, p=0.001).

Table 1
Baseline characteristics of properly monitored patients and comparison of SC group vs. non-SC group

References
1. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010; 53:105–113.

2. Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005; 270:13–19.

3. Chung WB, Youn HJ, Choi YS, Park CS, Oh YS, Chung WS, et al. The expression of cardiac ankyrin repeat protein in an animal model of adriamycin-induced cardiomyopathy. Korean Circ J. 2008; 38:455–461.

4. Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991; 266:1672–1677.

5. Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002; 86:1697–1700.

6. Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail. 2007; 9:409–414.

7. Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. 2010; 56:1644–1650.

8. Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992; 89(5 Pt 1):942–949.

9. Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009; 27:127–145.

10. van Dalen EC, van den Brug M, Caron HN, Kremer LC. Anthracycline-induced cardiotoxicity: comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur J Cancer. 2006; 42:3199–3205.

11. Jannazzo A, Hoffman J, Lutz M. Monitoring of anthracycline-induced cardiotoxicity. Ann Pharmacother. 2008; 42:99–104.

12. Nagy AC, Cserép Z, Tolnay E, Nagykálnai T, Forster T. Early diagnosis of chemotherapy-induced cardiomyopathy: a prospective tissue Doppler imaging study. Pathol Oncol Res. 2008; 14:69–77.

13. Jassal DS, Han SY, Hans C, Sharma A, Fang T, Ahmadie R, et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009; 22:418–424.

14. Di Lisi D, Bonura F, Macaione F, Peritore A, Meschisi M, Cuttitta F, et al. Chemotherapy-induced cardiotoxicity: role of the tissue Doppler in the early diagnosis of left ventricular dysfunction. Anticancer Drugs. 2011; 22:468–472.
15. Ho E, Brown A, Barrett P, Morgan RB, King G, Kennedy MJ, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010; 96:701–707.

16. Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011; 12:945–952.

17. Lightfoot JC, D'Agostino RB Jr, Hamilton CA, Jordan J, Torti FM, Kock ND, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010; 3:550–558.

18. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011; 107:1375–1380.

19. Bovelli D, Plataniotis G, Roila F. ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010; 21:Suppl 5. v277–v282.

20. Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008; 14:14–24.

21. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010; 55:213–220.
22. De Keyzer E, Kerkhove D, Van Camp G, De Sutter J, Achtergael W, Keymeulen B, et al. Screening for silent myocardial ischaemia in patients with type 2 diabetes mellitus: a quest to improve selection of the target screening population. Acta Cardiol. 2011; 66:715–720.

23. Perez EA, Suman VJ, Davidson NE, Kaufman PA, Martino S, Dakhil SR, et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J Clin Oncol. 2004; 22:3700–3704.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download