Abstract
Purpose
Mucins are members of the glycoprotein family expressed in benign and malignant epithelial cells. The aim of this study is to evaluate the relationships between the expression of mucins in breast ductal carcinoma and clinicopathologic parameters.
Methods
We constructed tumor microarrays based on 240 cases of invasive ductal carcinoma and 40 cases of ductal carcinoma in situ (DCIS) using formalin fixed, paraffin embedded tissues. We examined the expressions of MUC1, MUC2, MUC5AC, and MUC6 by immunohistochemistry.
Results
MUC1 demonstrated cytoplasmic, membranous, apical, and combinative expressions. Other mucins demonstrated cytoplasmic expression. In invasive ductal carcinoma, MUC1, MUC2, MUC5AC, and MUC6 were expressed in 93.6%, 6.2%, 4.8%, and 12.4% of cases, respectively; these rates were slightly, but not significantly, higher than observed in cases of DCIS. MUC1 expression was associated with estrogen receptor (ER) expression and negative MUC1 expression was associated with triple negativity. MUC6 expression was correlated with higher histologic grade, lymphatic invasion, lymph node metastasis, and HER2 positivity. No associations with any other clinicopathologic parameters were observed.
Conclusion
Most invasive ductal carcinomas of the breast express MUC1, and this expression is associated with ER expression. MUC6 expression is correlated with some clinicopathologic parameters that are indicators of poor prognosis. To evaluate the role of MUC6 as a potential biomarker, further studies are warranted.
Breast cancer is the most common malignancy in women, and therefore, much effort is devoted to identifying factors of prognosis and therapeutic significance. Mucins are high molecular weight glycoproteins with abundant O-linked carbohydrate chains. Their common domains are attached to serine or threonine residues that are O-glycosylated [1,2]. To date, several mucins have been identified: MUC1, MUC3, MUC4, MUC12, MUC13, and MUC17 are membrane bound types, and MUC2, MUC5AC, MUC5B, and MUC6 are secreted or gel forming types [3]. Alterations in mucin expression or glycosylation accompany the development of cancer and influence cellular growth, differentiation, transformation, adhesion, invasion, and immune surveillance [4]. The expressions of mucins in malignant tumors have been investigated most prominently in adenocarcinomas of organs, such as the pancreas, ovary, and stomach [5-9]. MUC1 is detected on the apical surfaces of most normal glandular epithelial tissues [10]. In contrast, MUC1 demonstrates variable localization in malignant tissues, including cytoplasmic and membrane expression [11]. Moreover, some studies suggest that the overexpression of MUC1 is associated with an increased metastasis rate [12,13]. MUC2 is a major gel-forming secretory mucin, which is expressed primarily in intestinal goblet cells and airway epithelium [14,15]. MUC2 demonstrates higher expression levels in mucinous carcinomas than in ductal carcinomas from several organs, including pancreas, breast, and prostate [16-19]. MUC5AC and MUC6 are gastric-type secretory mucins. MUC5AC is located mainly in the gastric mucosa of the cardia, fundus, and antrum, where as MUC6 is located in pyloric glands [20,21]. Interestingly, prior studies demonstrated a correlation between increased expression of MUC6 and less aggressive biological behavior in mucinous carcinoma of the breast [18].
The aims of the current study were to evaluate the expression patterns of various mucins in invasive ductal carcinoma of the breast and to determine the relationships between mucin expression and clinicopathologic parameters.
We studied 240 patients with invasive ductal carcinoma of no special type and 40 patients with ductal carcinoma in situ (DCIS), who underwent surgical resection without neoadjuvant therapy at the Kangbuk Samsung Hospital between January 2000 and December 2005. All studies were conducted with a prior approval of the Institutional Review Board (KBC12081). The median age of the patients was 47 years (range, 24-79 years) and the follow-up period ranged from less than 1 to 138 months. During the follow-up period, 53 patients developed distant metastasis and 22 patients died.
Hematoxylin and eosin (H&E) slides from all patients were reviewed by two pathologists (Do SI and Kim DH), and the histological data, such as T and N stage, lymphatic invasion and so on were reconfirmed. The discrepant cases were reviewed by the two pathologists together and a consensus result was achieved.
The surgical specimens were fixed in 10% buffered formalin, processed and embedded in paraffin using a standard protocol. All H&E-stained slides were reviewed and the most representative tumor area was carefully selected and marked on individual paraffin blocks. The most representative tissue core (2 mm diameter) was obtained from each tumor specimen. The tissue cores were arrayed in a recipient paraffin block manually by three pathologists (Chae SW, Kim DH, and Do SI).
Immunohistochemical stains were performed on 2 µm sectioned TMA blocks. Briefly, the sections were dehydrated, deparaffinized in xylene, and then rehydrated in a graded series of alcohol solutions. The antibodies used were MUC1 (1/200, Ma695 Clone; Novocastra, Newcastle, UK), MUC2 (1/200, Ccp58 Clone; Novocastra), MUC5AC (1/100, CLH2 Clone; Novocastra), MUC6 (1/100, CLH5 Clone: Novocastra), estrogen receptor (ER) (1/200, SP1 Clone; Labvision, Fremont, USA), progesterone receptor (PR) (1/200, PgR636 Clone; DAKO, Glostrup, Denmark), p53 (1/5,000, DO-7 Clone; DAKO), HER2 (1/200, SP3 Clone; Labvision), and CK5/6 (1/50, D5/16 B4 Clone; DAKO). Immunostaining was performed using a compact polymer method (Bond Intense Detection Kit; Leica Biosystems, Newcastle, UK).
MUC1 and MUC6 were considered positive if there was a positive expression in >30% of malignant cells. In the cases of MUC2 and MUC5AC, any expression in malignant cells was regarded as positive due to their low expression level. The Allred score was used to evaluate the ER and PR, and a score of 3 to 8 was considered positive. HER2 staining was scored according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines and positivity was defined by a score of 2+ and 3+ (complete membrane staining in more than 30% of malignant cells). As a prior study, CK5/6 was considered positive when there was an expression in >1% of malignant cells [22].
Statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, USA). The chi-square test and Fisher's exact tests were used to evaluate the associations between mucin expression and clinicopathologic parameters. The Kaplan-Meier estimator was used for the survival analysis. A p-value less than 0.05 was considered statistically significant.
MUC1 was expressed in various sites, such as apical, cytoplasmic, and membranous or a combination of cytoplasmic and membranous (Figure 1). Other mucins were expressed in the cytoplasm (Figure 2). In the assessable cases, DCIS demonstrated MUC1, MUC2, MUC5AC, and MUC6 expression in 88.6%, 2.6%, 5.6%, and 8.3% of total cases, respectively. The rates of MUC1, MUC2, MUC5AC, and MUC6 expression were 93.6%, 6.2%, 4.8%, and 12.4% in invasive ductal carcinoma, respectively. These rates were slightly higher than those in the cases of DCIS, however this difference did not reach a statistical significance (Table 1). Some values were missed due to tissue loss.
Clinicopathologic parameters included histologic grade (Bloom-Richardson grade), extensive intraductal carcinoma component (EIC), necrosis, lymphatic invasion, perineural invasion, tumor border, distant metastasis, T stage, N stage, ER, PR, HER2, CK5/6 (basal like carcinoma) expression, triple negativity, Paget's disease, and the overall survival. Regardless of subcellular localization, MUC1 expression demonstrated associations with positive ER and PR expression (p=0.002 and p=0.012, respectively) and was inversely associated with triple negativity (p<0.001). There were no associations between MUC1 expression and other clinicopathologic parameters. In the cases of MUC1 expression classified by subcellular localization sites, apical, cytoplasmic, and membranous or a combination of cytoplasmic and membranous, expression was observed in 4.1%, 62.2%, and 27.3% of invasive ductal carcinoma cases, respectively (Table 2). There were no specific differences between MUC1 expression sites and clinicopathologic parameters. MUC2 and MUC5AC were expressed in 6.2% and 4.8% of invasive ductal carcinomas, respectively, and were not associated with any clinicopathologic variables. MUC6 expression was correlated with a higher histologic grade, presence of lymphatic invasion, and a higher N stage (p=0.040, p=0.022, and p=0.042, respectively) (Table 3, Supplementary Table 1, available from http://www.ejbc.kr). With the exception of these differences, no other associations were found with the other parameters and MUC6 expression. The Kaplan-Meier survival analysis revealed no prognostic significance of the overall survival for MUC1, MUC2, MUC5AC, or MUC6 expression (p=0.463, p=0.970, p=0.408, and p=0.237, respectively) (Figure 3). Any statistical correlation between MUC2, MUC5AC, or MUC6 expression and disease free survival was not shown (p=0.611, p=0.550, p=0.896, and p=0.523, respectively).
In the current study, we investigated different mucin expression patterns and their associations with clinicopathologic parameters in human breast cancer. In a previous study, even though benign breast showed a different expression pattern, DCIS and invasive ductal carcinoma showed a similar expression pattern [23]; moreover we also observed a similar expression pattern between DCIS and invasive ductal carcinoma. Hence, we evaluated the expression pattern of DCIS and invasive ductal carcinoma with the same criteria. However, there was no statistical correlation between the expression of mucins with DCIS and invasive ductal carcinoma. Consistent with the previous study demonstrating about 90% of MUC1 expression [23,24], we observed MUC1 expression in the majority of invasive ductal carcinoma cases (93.6%). Rakha et al. [24] and van der Vegt et al. [25] found that apical MUC1 expression was associated with superior outcomes. Different from the study of Rakha et al. [24] showing apical staining in 4.5% of cases, another study of MUC1 in Korean breast cancer patients showed only one case of apical expression in 244 cases (0.4%); further the evaluation of prognostic correlation was not conducted [23]. Even though we observed in 4.1% of apical expression, the current study did not demonstrate any correlations between MUC1 expression sites and clinicopathologic parameters. The differences between the results of previous studies and the present study are controvertible; thus further study might be required. Regardless of subcellular localization, MUC1 expression was associated with ER and with PR positivity and nontriple negativity. In the cases of MUC2 and MUC5AC, any expression in malignant cells was regarded as positive due to their low expression level, and they showed a similar positivity rate with the previous study [18]. MUC2 and MUC5 expressions were not correlated with any prognostic factors. A previous study of mucinous carcinoma demonstrated an association between better prognosis and MUC2 expression. However, the expression rate was significantly higher in mucinous carcinoma, as it plays a role in barrier formation in mucinous carcinoma: however, its role in invasive ductal carcinoma is not clear. In the current study, MUC6 expression was observed in 12.4% of invasive ductal carcinoma cases, and there were correlations between MUC6 expression and poor prognostic factors, such as a higher histologic grade, lymphatic invasion, and higher N stage. Previous studies of MUC6 expression in breast cancer by Pereira et al. [26] and Rakha et al. [24] detected MUC6 expression in approximately 20% to 23% of cases. The differences in MUC6 expression proportions between the current study and the previous two studies may be due to different criteria for positivity. We defined positivity as when more than 30% of cancer cells expressed MUC6, where as the prior studies used a cutoff value of more than 5%. Because we failed to attain a valuable receiver operating characteristic (ROC) curve to evaluate the relationship between the overall survival and MUC6, for the determination of the optimal cutoff value, we have evaluated a 5% interval and found that the value of 30% shows a relationship with some clinicopathologic parameters. Additionally, the previous studies did not demonstrate any correlations between MUC6 expression and clinicopathologic parameters. These differences may also be partially explained by the different criteria for positivity. In addition, because all cases of this study are from Asian women and all previous studies were from Western women, genetic differences should be considered and a molecular level study might be needed. Moreover, a previous study investigating MUC6 expression in mucinous carcinoma of the breast by Matsukita et al. [18] found higher MUC6 expression in mucinous carcinomas (71%) and suggested that MUC6 expression is associated with superior outcomes. These authors explained that, as a gel-forming secretory mucin, MUC6 may act as a barrier to cancer cell extension, resulting in a better prognosis. However, as the number of cases in their study was only 17 cases and relatively limited, larger studies would be helpful to confirm this result. Even though MUC6 expression was observed in the current study, we did not observe a mucin barrier in invasive ductal carcinoma. The role of MUC6 may differ between invasive ductal carcinoma and mucinous carcinoma of the breast. We note that our study has some limitations. First, the representative tumor areas may not have been evaluated, because mucins evaluation was carried out only on one cut surface of each tumor specimen without an analysis of whole mount section. Second, the result of HER2 has a limitation because the evaluation was performed via immunohistochemistry staining without the evaluation of fluorescence in situ hybridization or silver in situ hybridization.
In summary, MUC1 is expressed in most cases of invasive ductal carcinoma in the breast and is associated with hormonal receptor expression; however, it is not associated with clinicopathologic parameters. Among the various mucins studied, MUC6 expression was associated with parameters known as poor prognostic factors such as grade, lymphatic invasion and N stage, when more restrictive criteria were applied. Further studies, including studies at the genetic level, are needed in order to clarify the differences in the results between the current and previous studies.
Figures and Tables
Figure 1
MUC1 expression with cytoplasmic, membranous and apical or combinative location. MUC1 expression shows variable subcellular locations, such as cytoplasmic (A), membranous (B), apical (C), and combinative cytoplasmic and membranous (D) (immunohistochemical staining, ×200).
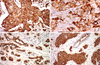
Figure 2
MUC2, MUC5AC, and MUC6 expression. MUC2 (A), MUC5AC (B), and MUC6 (C) show cytoplasm expression (immunohistochemical staining, ×200).
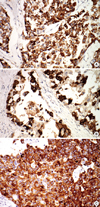
Figure 3
Kaplan-Meier survival analysis results for MUC1 (A), MUC2 (B), MUC5AC (C), and MUC6 (D). Statistical significance for survival is not observed in any of the mucins.

Table 1
MUC1, MUC2, MUC5AC, and MUC6 expression in invasive ductal carcinoma and ductal carcinoma in situ

Table 3
Mucin expression and clinicopathological parameters of invasive ductal carcinomas

Some values were missed due to tissue loss. Other clinicopathological parameters which did not show statistical value are in Supplementary Table.
ER=estrogen receptor; PR=progesterone receptor; +=positive or present; -=negative or absent.
*Linear by linear test; †Chi-square test and Fisher's exact test.
ACKNOWLEDGEMENTS
This study was supported by Sungkyunkwan University Foundation for Corporate Collaboration.
References
2. Kim YS, Gum J Jr, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996; 13:693–707.

3. Fowler J, Vinall L, Swallow D. Polymorphism of the human muc genes. Front Biosci. 2001; 6:D1207–D1215.

4. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004; 4:45–60.

5. Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993; 71:2191–2199.

6. Tashiro Y, Yonezawa S, Kim YS, Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human ovarian tumors. Hum Pathol. 1994; 25:364–372.

7. Utsunomiya T, Yonezawa S, Sakamoto H, Kitamura H, Hokita S, Aiko T, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998; 4:2605–2614.
8. Yamashita K, Yonezawa S, Tanaka S, Shirahama H, Sakoda K, Imai K, et al. Immunohistochemical study of mucin carbohydrates and core proteins in hepatolithiasis and cholangiocarcinoma. Int J Cancer. 1993; 55:82–91.

9. Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999; 49:45–54.

10. Patton S, Gendler SJ, Spicer AP. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta. 1995; 1241:407–423.

11. Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer. 2001; 91:1973–1982.

12. Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995; 129:255–265.

13. Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003; 63:5011–5020.
14. Gum JR Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994; 269:2440–2446.

15. Chang SK, Dohrman AF, Basbaum CB, Ho SB, Tsuda T, Toribara NW, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994; 107:28–36.

16. Adsay NV, Merati K, Nassar H, Shia J, Sarkar F, Pierson CR, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003; 27:571–578.
17. Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, Conlon KC, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001; 25:26–42.

18. Matsukita S, Nomoto M, Kitajima S, Tanaka S, Goto M, Irimura T, et al. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: comparison with invasive ductal carcinoma. Histopathology. 2003; 42:26–36.

19. Osunkoya AO, Adsay NV, Cohen C, Epstein JI, Smith SL. MUC2 expression in primary mucinous and nonmucinous adenocarcinoma of the prostate: an analysis of 50 cases on radical prostatectomy. Mod Pathol. 2008; 21:789–794.

20. De Bolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995; 109:723–734.

21. Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995; 55:2681–2690.
22. Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011; 24:157–167.

23. Chang E, Lee E, Yoo C, Oh SJ, Kim JS, Kang C. Cellular localization of MUC1 in benign and malignant breast lesions with the histological correlation and the prognostic significance. J Breast Cancer. 2005; 8:150–156.

24. Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005; 18:1295–1304.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download