Abstract
Purpose
Breast cancer is one of the most frequent malignancies in Korean women, and its incidence is increasing at a rapid rate. Since 1996, the Korean Breast Cancer Society has collected nationwide breast cancer data using an online registration program and analyzed the data biennial. The purpose of this study was to evaluate the characteristics of Korean breast cancer and to analyze changes in these characteristics over the period of time.
Methods
Data were collected from 41 medical schools (74 hospitals), 24 general hospitals, and 6 private clinics. Data on the total number, gender, and age of newly-diagnosed breast cancer patients were collected through a questionnaire. Additional data were collected and analyzed from the online database.
Results
In 2010, 16,398 patients in Korea were newly diagnosed with breast cancer. The crude incidence rate of female breast cancer was 67.2 cases per 100,000, and the median age at diagnosis was 49 years. The incidence of breast cancer was highest in patients aged between 40 and 49 years. Since 1996, there has been a significant increase in the proportion of early-stage cancers (detected in stage 1 or 2), the percentage of estrogen receptor-positive cancers, and in the proportion of patients receiving breast-conserving surgery.
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among females, accounting for 23% of cancer cases and 14% of cancer deaths worldwide [1]. Trends in the incidence and death rates due to breast cancer differ among countries [1-3]. Korea has one of the lowest breast cancer incidence rates (43.8 per 100,000 women-years in 2009) [4,5]. However, the incidence rate is rising rapidly and breast cancer is the second most common cancer among women in Korea [6-12]. To define changing patterns in the clinical characteristics of breast cancer in Korean patients, since 1996, the Korean Breast Cancer Society (KBCS) has compiled data on Korean breast cancer patients. The purpose of this study was to analyze chronological changes in breast cancer characteristics and risk factors in Korean women during the period from 1996 to 2010.
Data were collected on women with newly-diagnosed primary breast cancer treated throughout Korea from January 1st to December 31st, 2010. Thirty-eight of the 41 medical schools in Korea, as well as 24 general hospitals and 6 private hospitals and special clinics, participated in the survey, totaling 104 hospitals and clinics. A questionnaire asking for the total number of patients and for each patient's gender and age group was forwarded to physicians from each hospital.
Other analyses were performed using the patient population from the online database. The data included parameters such as clinical symptoms, physical findings (palpability, location), test findings (method of confirmation of cancer diagnosis and presurgical blood test findings), surgical findings (surgical method, complications, and reconstruction method), pathological findings (American Joint Committee on Cancer [AJCC] stage, tumor, node, metastasis [TNM] stage, histological categorization, hormone receptor status, and human epidermal growth factor receptor 2 [HER2] positivity), patient history, and reproductive risk factors, among others.
The number of newly-diagnosed breast cancer patients in 2010 was 16,398 (Table 1). Diagnoses were made at university hospitals in 14,214 cases (86.7%), at general hospitals in 1,937 cases (11.8%), and at private clinics in 247 cases (1.5%), respectively. The youngest of the patients was 3.6 years old and the oldest was 96 years old, with a median age of 49 years. The age range with the largest number of newly diagnosed cases was 40 to 49 years of age (6,088 cases, or 37.1%), followed by 50 to 59 years of age (4,772, or 26.1% of cases). The number of male breast cancer patients was 49 (0.3%), with most of the patients diagnosed between 40 and 49 years of age (n=17) (Table 1).
A painless lump in the breast was the most common symptom, being reported by 3,943 (56.0%) of the 7,035 patients enrolled in the online directory in 2010. The number of asymptomatic patients diagnosed on routine screening was 2,298 (32.7%) (Table 2).
The most frequent method used to confirm the diagnosis of breast cancer was core needle biopsy, which was performed in 81.3% of cases (Table 3).
Among the 7,079 patients, whose surgical methods could be confirmed, breast conservation surgery was most common in 4,293 cases (60.6%) followed by modified radical mastectomy in 2,689 cases (38.0%) (Table 4). Since the publication of the 7th edition of the AJCC, stage I breast tumors have been subdivided into stage IA and stage IB. Stage IB includes small tumors (TI) with lymph node micrometastases (N1mi).
In the present investigation, stage could be determined in 7,078 cases, 39.1% of which were classified as stage I, followed by 33.6% at stage II at 33.6% and 12.8% at stage 0 (Table 5).
Among 6,257 patients whose pathologic results could be confirmed, invasive ductal carcinoma was the most common diagnosis, with 5,197 cases (83.1%), followed by ductal carcinoma in situ (DCIS) in 753 patients (12.0%), and invasive lobular carcinoma in 188 patients (3.0%) (Table 6).
According to the World Health Organization (WHO) classification system, invasive ductal carcinoma not otherwise specified (NOS) was the most frequent, with 4,112 cases (82.1%), followed by invasive ductal carcinoma, with 380 cases (7.6%) (Table 7). Tumor size could be confirmed in 7,079 cases, 51.5% of which were of size T1, and 29.3% of which were of size T2 (Table 8).
N0 was the most common nodal stage (4,375 cases, 69.2%), followed by N1 (1,337 cases, 21.1%), N2 (398 cases, 6.3%), and N3 (215 cases, 3.4%), respectively (Table 9).
The percentages of tumors with positive estrogen or progesterone receptor expression were 69.9% and 58.3%, respectively. For the expression of c-erbB-2, 43.0% of cases were immunohistochemically negative, and 17.3% were classified as 1+, 17.8% as 2+, and 21.5% as 3+ (Table 10). With respect to p53 expression, 67.1% of cases were negative, 9.5% were 1+, 6.9% were 2+, and 16.3% were 3+ (Table 10).
Breast cancer incidence in Korea has been rising steadily. During the 14-year period from 1996 to 2010, the number breast cancer patients registered with the KBCS has risen from 3,801 to 16,398 (331.4% rise; R2=0.973, p<0.001) (Figure 1).
The crude incidence rate of Korean female breast cancer cases in 2010, including carcinoma in situ, was calculated at 67.2 per 100,000, a significant increase from 57.4 in 2008.
Among all breast cancers, infiltrating cancer occurred at a rate of 58.5 new cases per 100,000 women, and carcinoma in situ occurred at a rate of 8.7 per 100,000, thus confirming an increase in the crude incidence rate for both (Figure 2).
The age-specific crude incidence rate was the highest in women aged 40 to 49 years, with 147.9 cases per 100,000 women, followed by 144.2 for women aged 50 to 59 years, 108.3 for women aged 60 to 69 years, 55.8 for women aged 70 to 79 years, and 52.7 for women aged 30 to 39 years (Figure 3).
The age-specific incidence graph was shifted to the right when compared to the previous data, indicating an increase in the age of onset. Furthermore, there were differences in the degree of the rise in incidence among the different age groups. For women in their 60s, the crude incidence rate increased 3.4 times (from 31.5 per 100,000 in 1998 to 108.3 in 2010), and for women in their 70s, the rate increased 3.8 times (from 14.6 in 1998 to 55.8 in 2010). The incidence increased 2.3 times for women in their 30s, 2.5 for women in their 40s, and 2.6 times for women in their 50s, indicating that between 1998 and 2010, breast cancer incidence increased the most in older age groups. The median age of diagnosis therefore also increased over time, from 46 in 1996 to 49 in 2010, and the ratio of postmenopausal to premenopausal females also rose slightly, from 39.1% in 1996 to 48.7% in 2010 (a 24.6% increase; R2=0.802, p=0.003) (Figure 4).
As far as the clinical manifestation of disease is concerned, the percentage of incidental asymptomatic breast cancer detected at screening experienced a steep increase from 6.4% in 1996 to 32.7% in 2010 (a 410.9% increase; R2=0.933, p<0.001) (Table 11).
The proportion of breast cancers diagnosed at the early stages (stages 0 and I according to the AJCC 6th staging system) steadily increased from 1996 to 2010. The proportion of cancers diagnosed as stage 0 rose from 4.2% to 13.0% (209.5% increase; R2=0.970, p<0.001), and the proportion of newly-diagnosed cancers in stage I rose from 19.6% to 39.5% (101.5% increase; R2=0.896, p<0.001). However, the percentage of stage II to IV cancers decreased from 76.2% to 47.4% (37.8% decrease; R2=0961, p<0.001) (Figure 5).
Figure 6 shows changes in the surgical methods employed for treatment of breast cancer from 1996 to 2010. A steady decrease in mastectomy from 79.7% in 1996 to 38.0% in 2010 (52.3% decrease; R2=0.981, p<0.001) was noted, whereas the percentage of breast-conserving operations accordingly increased, from 18.7% to 60.6% (224.1% rise; R2=0.987, p<0.001).
By means of histological type, DCIS increased from 4.9% of all breast cancer cases in 1996 to 12.0% in 2010 (144.9% rise; R2=0.864, p<0.001), whereas other cancer types steadily decreased (Table 12).
The rate of estrogen receptor (ER)-positive breast cancer increased from 49.6% in 1996 to 70.0% in 2010 (41.1% rise; R2=0.962, p<0.001) (Figure 7).
Table 13 shows changes in the proportion of patients according to reproductive factors among Korean women from 1996 to 2010. The percentages of newly-diagnosed women diagnosed with these known risk factors increased from 1996 to 2010.
Global breast cancer incidence has experienced an increase from 641,000 cases in 1980 (95% uncertainty interval; range, 610,000-750,000) to 1,643,000 cases in 2010 (range, 1,421,000-1,782,000) [2]. Trends in breast cancer incidence differ between Asian and Western countries. Generally, incidence rates are lower in Korea than in the Western countries. However, at present, there is a rapid increase in the incidence rate in Korea than in Western countries [1-3,13].
The reasons for the rising trends are not completely understood but likely reflect birth-cohort effects, changes in reproductive factors, and the introduction of screening programs, all of which have contributed to the increase in breast cancer incidence in Asian women [4]. Based on the nationwide incidence data collected by the Korean Central Cancer Registry (KCCR) of the Korea Cancer Center, the number of new breast cancer cases in South Korea has demonstrated a steady rise over time [6-12].
The age-standardized incidence rate of breast cancer in Korea in 2009 was 43.8 per 100,000 [14]. From 1999 to 2009, the annual age-adjusted breast cancer incidence rate was 6.3% in Korean women [14]. This is higher than the worldwide average of 3.1% [2].
Breast cancer demographic studies from China, Taiwan, India, Japan, South Korea, Sweden, Canada, and the United States revealed a striking difference in peak incidence, which is between 40 and 50 years of age in Asian countries and between 60 and 70 years of age in Western countries [13]. In Korea, however, the median age of newly-diagnosed patients in 2010 was found to be 49 years, which was slightly higher than in the past, but is still more than 10 years lower than in the United States. The age-specific incidence rate in Korea was 147.9 cases per 100,000 for women in their 40s, 144.2 for those in their 50s, 108.3 for those in their 60s, and 55.8 for those in their 70s, again showing a decreasing trend in cancer incidence with increasing age.
In the United States, the median age of diagnosis for female breast cancer patients between 2005 and 2009 was 61 years. The age-specific incidence rate was 205.7 per 100,000 for women in their 40s, 326.6 for those in their 50s, 478.4 for those in their 60s, and 520.4 for those in their 70s, indicating that in the United States, the incidence rate increased along with increase in age (Figure 8) [15].
In Japan, the age distribution was similar to that in Korea in the past; however, recent data demonstrates that the incidence rates for women in their 50s and 60s have increased. Incidence gradually increased for women in their 40s to their 60s, and then slightly decreased for women in their 70s, which is a different trend from that seen in Korea (Figure 8) [16].
As for breast cancer discovery, asymptomatic cases detected upon routine screening comprised only 6.4% of all new cases in 1996, but this percentage increased by more than 5-fold (to 32.7%) in 2010, indicating that public awareness campaigns and increased access to screening programs have been successful to some extent. Furthermore, more cancers are being diagnosed at early stages. The percentage of cancers diagnosed at stage 0 has consistently increased since 1996 and reached 13.0% in 2010 (the highest rate ever reported). However, the ratio of all early-stage cancers (stages 0 and I) did not change significantly (45.2% in 2004, 47.5% in 2006, and 47.2% in 2008). Therefore, the efficacy of breast screening needs to be evaluated in future investigations.
In all randomized trials of breast cancer screening in which a decrease in breast cancer mortality was observed, there was a decrease in the risk of being diagnosed with an advanced breast cancer [17].
One of the noticeable findings in this study was that the ratio of breast conservation surgery surpassed that of mastectomy. This ratio was only 18.7% in 1996; however, it has constantly increased since then, reaching 60.6% in 2010. This is more than 20% higher than the mastectomy rate, which is 38.0%. Early detection and neoadjuvant chemotherapy may have contributed to the increase in number of patients with operable breast cancer, who are candidates for breast-conserving surgery.
Similar increase in trends in breast-conserving surgery has been observed in Japan also [18]. However, different trends in surgical methods were found in the United States. Habermann et al. [19] reported that the proportion of women treated with mastectomy significantly decreased, from 41% in 2000 to 37% in 2006. Interestingly, the mastectomy proportion slightly increased from 2005 to 2006, perhaps predicting future trends in the United States.
In this study, we revealed a notable recent increase in the proportion of ER-positive breast cancer (Figure 7).
A similar trend was observed in other countries also. DeSantis et al. [20] recently noted that incidence rates of ER-positive breast cancer in American women had stabilized (overall) or increased (in the subset of women aged 40-49 years) from 2003 to 2007. Yamashita et al. [21] reported that the increase in Japanese breast cancer incidence mostly reflects an increase in the ER-positive subtype, especially in women aged 50 years or younger.
There are several reasons for the increase in ER-positive breast cancer. Western populations are more likely to have factors that increase the risk for the luminal A type [22].
The Western lifestyle, including diet and reproductive factors such as early age at menarche, late age at menopause, delay in marriage, having fewer children later in life, and changes in infant feeding patterns, are some specific trends observed in Korea. Thus, changing lifestyles might increase the risk of breast cancer, especially in premenopausal Korean women.
Yip et al. [23] recently reported that ER positivity was significantly associated with early stage (I, II) and low grade.
Recent changes in breast cancer incidence overall reflect divergent trends in ER-positive and ER-negative cancers. If current trends continue, the incidence of ER-positive breast cancers will increase, the incidence of ER-negative breast cancers will continue to decrease, and the overall incidence of breast cancer will remain steady [24]. There are some limitations in this study. Since the data reported in this paper is based on a survey, there are some possible omissions. These data may therefore be slightly different to that of the National Cancer Registry System. In addition, data regarding the survival rate is unknown. While the total number, gender, and age distribution of patients who received operations in 2010 are based on survey data, clinical manifestations, diagnostic methods, surgical methods, biomarkers, and risk factors; the rest of the data tables are based on registered data of the online registration system. For that reason, there was a difference in patient numbers. However, this paper is clearly based on actual data. We also believe that even if there was a minor difference, the data still broadly represents overall outcomes of the patients.
The results propose that the clinical characteristics of breast cancer in Korean patients are slowly changing when compared to the patterns in Western countries, and the incidence of breast cancer in Korea will continue to rise. It is also proposed that the characteristics of breast cancer in the Korean population must be studied using available online databases.
Figures and Tables
Figure 1
Number of newly-diagnosed breast cancer patients according to the Korean Breast Cancer Society survey.

Figure 3
Age-specific crude incidence of Korean female breast cancer. *New diagnoses number per 100,000 women.

Figure 4
Trends in the median age of Korean breast cancer patients and in the ratio of postmenopausal to premenopausal women at the time of diagnosis.
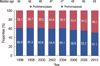
Figure 5
Breast cancer in Korean women by stage, according to the American Joint Committee on Cancer classification system (6th edition).
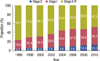
Figure 8
Comparison of age-specific crude incidence rates. *New breast cancer cases per 100,000 women: based on †cancer statistics in Japan (2007); ‡SEER 18 data (2005-2009).

ACKNOWLEDGEMENTS
The Korean Breast Cancer Society thanks the following members who participated in this national study: Kang SY, Kang SH, Kang YJ, Kang EY, Kang HS, Kang HJ, Go BG, Ko JW, Ko CD, Kwak BS, Kwak JH, Kwak HN, Kim KS, Kim KC, Kim DI, Kim SY, Kim SW (4), Kim SY, Kim SW, Kim SJ, Kim SK, Kim YH, Kim WW (2), Kim JY, Kim JI, Kim LS, Kim JS (2), Kim JR, Kim TH, Kim HK, Kim HS (2), Kim HJ, Nam SJ, Nam SY, Nam YH, Noh DY, Ryu WS, Moon BI, Moon HG, Min JW, Park KS, Park MH, Park SH, Park ST, Park YL, Park WC, Park JP (2), Park HL, Park HK. Park HB, Park HY, Bae YT, Bae JW, Paik NS, Baek JM, Bong JG, Suh YJ, Sun WY, Son KY, Son GS, Son BH, Song BJ, Song YJ (2), Song JY, Shin SH, Shin JH, Shin HJ, Shin HC, An MH, Ahn SK, Yang GS, Yang JH, Yom CK, Oh SJ, Yoo YB, Yoon DS, Yun MY, Yoon JH, Lee KS, Lee KP, Lee DS (2), Lee MR, Lee MH, Lee SJ (2), Lee SY, Lee SH, Lee AB, Lee YL, Lee WH, Lee EJ, Lee YO, Lee IK, Lee JA, Lee JE, Lee JW (2), Lee JY (3), Lee JJ, Lee JS (2), Lee JH (4), Lee CH, Lee HD, Lee HY, Lim YA, Lim WS, Lim JY, Lim CW, Chang MC, Chang ES, Chang JN, Jun SY, Jeon YW, Jeon YS, Jeon CW, Chung MS, Jung PJ, Jung BH, Jung SS, Jung SY, Jung YS (2), Jung YJ, Jung EJ, Chung IY, Jeong J, Jung JH, Jeong HY, Jo BH, Cho SH, Cho YU, Cho JY, Cho JS, Chae BJ, Choi MS, Choi SY, Choi SH, Choi YJ, Choi JH, Han JW, Han SA, Han SW, Han AR, Han WS, Hwang KT, Hwang SH.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011. 61:69–90.

2. Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011. 378:1461–1484.

4. Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010. 21:1777–1785.

5. National Cancer Center. Report on the Statistics for National Cancer Incidence 2009. 2011. Goyang: National Cancer Center.
6. Korean Breast Cancer Society. Korean breast cancer data of 1996. J Korean Surg Soc. 1998. 55:621–635.
7. Korean Breast Cancer Society. Korean breast cancer data of 1997. J Korean Cancer Assoc. 1999. 31:1202–1209.
8. Korean Breast Cancer Society. Clinical characteristics of breast cancer patients in Korea in year 2000. J Korean Breast Cancer Soc. 2002. 5:217–224.
9. The Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2004 using breast cancer registration program. J Breast Cancer. 2006. 9:151–161.
10. Ahn SH, Yoo KY. Korean Breast Cancer Society. Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996-2004. Breast Cancer Res Treat. 2006. 99:209–214.

11. Ko SS. Korean Breast Cancer Society. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years (1996-2006) using nationwide breast cancer registration on-line program: biannual update. J Surg Oncol. 2008. 98:318–323.

12. Jung YS, Na KY, Kim KS, Ahn SH, Lee SJ, Park HK, et al. Nation-wide Korean breast cancer data from 2008 using the breast cancer registration program. J Breast Cancer. 2011. 14:229–236.

13. Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010. 34:2308–2324.

14. National Cancer Center. Report on the Statistics for National Cancer Incidence 1999-2009. 2012. Goyang: National Cancer Center.
15. National Cancer Institute. SEER cancer statistics review, 1975-2009. Accessed October 31st, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/index.html.
16. Lambe M, Wigertz A, Holmqvist M, Adolfsson J, Bardage C, Fornander T, et al. Reductions in use of hormone replacement therapy: effects on Swedish breast cancer incidence trends only seen after several years. Breast Cancer Res Treat. 2010. 121:679–683.

17. Autier P, Hery C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009. 27:5919–5923.

18. Saji S, Hiraoka M, Tokuda Y, Fukui N, Ikeda T. Trends in local therapy application for early breast cancer patients in the Japanese Breast Cancer Society Breast Cancer Registry during 2004-2009. Breast Cancer. 2012. 19:1–3.

19. Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010. 28:3437–3441.

20. DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011. 20:733–739.

21. Yamashita H, Iwase H, Toyama T, Takahashi S, Sugiura H, Yoshimoto N, et al. Estrogen receptor-positive breast cancer in Japanese women: trends in incidence, characteristics, and prognosis. Ann Oncol. 2011. 22:1318–1325.

22. Devi CR, Tang TS, Corbex M. Incidence and risk factors for breast cancer subtypes in three distinct South-East Asian ethnic groups: Chinese, Malay and natives of Sarawak, Malaysia. Int J Cancer. 2012. 131:2869–2877.





 PDF
PDF ePub
ePub Citation
Citation Print
Print















 XML Download
XML Download