Abstract
Oncoplastic breast surgery has become a popular choice of treatment for breast reconstruction after mastectomy. There are two different techniques in oncoplastic surgery depending on the volume of the excised breast tissue. One is the volume displacement procedure, which combines resection with a variety of different breast-reshaping and breast-reduction techniques; the other is the volume replacement procedure in which the volume of excised breast tissue is replaced with autologous tissue. In this study, current authors performed various volume replacement techniques based on the weight of the excised tumor and its margin of resection. We used a latissimus dorsi myocutaneous flap for cases in which the resection mass was greater than 150 g, and for cases in which the resection mass was less than 150 g, we used a regional flap, such as a lateral thoracodorsal flap, a thoracoepigastric flap, or perforator flaps, such as an intercostal artery perforator flap or a thoracodorsal artery perforator flap. In the patients with small to moderate-sized breasts, when a postoperative deformity is expected due to a large-volume tumor resection, the replacement of non-breast tissue is required. Many of whom have small breasts, oncoplastic volume replacement techniques in breast-conserving surgery allow an extensive tumor excision without concern of compromising the cosmetic outcome and can be reliable and useful techniques with satisfactory aesthetic results.
Oncoplastic breast surgery has become a popular choice for breast reconstruction after mastectomy. It is a plastic surgical procedure in which the breast is redesigned using the residual or the adjacent breast tissue following excision of the breast cancer. Two different oncoplastic surgery techniques exist, and the choice of which technique to use depends on the volume of the excised breast tissue. One is the volume displacement procedure, which combines resection with a variety of different breast-reshaping and breast-reduction techniques; the other is the volume replacement procedure in which the volume of excised breast tissue is replaced with autologous tissue [1]. According to the volume replacement technique, when there is an insufficient volume of remaining breast tissue to perform a reconstruction, autologous tissues are imported and used as tissue flaps. These procedures can retain the volume and shape of the breast and avoid contralateral breast surgery. However, these techniques can be more complex and require a donor site and increased recovery time following autologous tissue harvesting [2].
In this study, current authors provide a comprehensive and detailed overview of various volume replacement techniques, including an adipofascial flap, a lateral thoracodorsal flap, a thoracoepigastric flap, an intercostal artery perforator (ICAP) flap, a thoracodorsal artery perforator (TDAP) flap, and a latissimus dorsi (LD) myocutaneous flap, for partial breast reconstruction in patients with small- to moderate-sized breasts.
To select the proper oncoplastic procedure, it is important to consider multiple factors including breast size/ptosis, excised volume and the location of the breast tumor. As demonstrated in a Caucasian population, for cases in which the breast size is relatively large, the amount of residual tissue following tumor excision is sufficient. Therefore, satisfactory cosmetic outcomes can be obtained by glandular reshaping and reduction mammoplasty as described in part 1. For cases in which the breast size is relatively small, as demonstrated in an Asian population, volume displacement techniques can also be performed after the removal of a small-sized defect. However, for cases in which the area of the defect is considered to be larger than moderate, satisfactory cosmetic outcomes only can be obtained so long as the defect area should be restored using a volume replacement procedure with adjacent tissue flaps [3].
In the present study, the proper technique was primarily selected according to the excised volume of the breast. The current authors performed various volume replacement techniques based on the weight of the excised tumor and its margin of resection. We used a LD myocutaneous flap for cases in which the resection mass was greater than 150 g, and for cases in which the resection mass was less than 150 g, we used a regional flap, such as a lateral thoracodorsal flap, a thoracoepigastric flap, or perforator flaps, such as an ICAP flap or TDAP flap (Figure 1). When the partial mastectomy defect is significant with minimal residual breast tissue, a decision is required regarding whether to complete the mastectomy and perform total breast reconstruction, for both cosmetic and oncological reasons [4]. The location of the tumor is an important factor involved in selecting the appropriate oncoplastic procedure. The LD myocutaneous flap technique is commonly used for lateral and central defects, even for medial defects. The thoracodorsal perforator flap can be easily used for defects in the lateral and central regions of the breast. The lateral intercostal perforator flap is an alternative choice for lateral and inferior breast defects. The anterior ICAP flap and the thoracoepigastric flap are appropriate for close defects that extend across the inferior or medial quadrants of the breast [4,5].
Volume replacement procedures can be implemented for immediate or delayed reconstruction. Benefits of immediate reconstruction include the avoidance of scar formation, radiation change in the breast, a second operation and the provision of superior aesthetic outcomes with fewer complications. However, there is a possibility that the final pathology report will show a positive margin and the local recurrence of malignancy. In those patients who are indicated for immediate partial reconstruction, it is important to reduce the incidence of positive margins, which can be done through the careful selection of patients. To select the appropriate volume replacement procedure, tumor size, location, and nodal status should be considered. When immediate reconstruction is performed, preoperative and intraoperative assessment of margins radiographically, macroscopically, cytologically, and pathologically should be performed. During the preoperative workup, digital mammography, breast ultrasound and breast magnetic resonance imaging should be performed and a marking (drawing) on the skin and parenchyma of the breast to identify the region to be excised should be made after enough discussion with the radiologist, breast surgeon and plastic surgeon. Then, the decision of the optimal surgical method is made, and breast surgery proceeds by the breast surgeon and the plastic surgeon [4,6-8].
Before surgery, we marked the cancer location, inframammary fold, and flap design with patients in the standing position. Under general anesthesia, all patients had undergone wide local excision with a safety margin of at least 1-2 cm; the skin overlying the tumor was excised only if it was included in the safety margin, such as for a superficial tumor location or if recent excisional or incisional biopsy was performed. Subsequently, axillary dissection is performed. The intraoperative specimen was routinely sent to the histopathology department after appropriate labeling to assess the margins by frozen section. Further excision of the concerning margin was performed.
The adipofascial flap can be harvested from the area below the inframammary fold or the thoracodorsal area. After wide local excision, the skin of the desired area is dissected through subcutaneous fat to a depth of 3 or 4 mm. After sufficient dissection, the subcutaneous fat and anterior sheath of the rectus abdominis muscle, or fascia of the LD muscle, is cut semicircularly around the intended area. Harvesting the fascia with the adipofascial flap makes the harvested flap firm. If the volume of the elevated flap is insufficient, we can get the additional volume by including muscles underlying the adipofascial flap. The elevated flap is gathered at the site of the breast tissue defect to reconstruct the breast mound and is fixed to the chest wall. After modeling the breast mound with the adipofascial flap, the skin is sutured, and a negative pressure drain is placed in the dissected area (Figure 2) [9].
This flap is useful for patients who have sufficient subcutaneous tissue at the inframammary region or thoracodorsal area and whose contralateral breast is not too large. Even if the patient is not obese, this flap can easily be used to reconstruct defects in the lower or lateral quadrant of the breast [10].
The lateral thoracodorsal flap is a wedge-shaped transposition flap. The axis of the flap is located in the lateral extension of the inframammary fold. The superior border of the flap begins just medial to the anterior axillary fold and extends laterally. The inferior border is curved; thus, it begins a few centimeters lateral to the superior border. It is important to determine the available amount of skin and the subcutaneous fat tissue in the lateral thoracic region, which can be measured by pinching the skin and soft tissue between the thumb and the index finger without transferring tension to the mammary region. The skin, fat and the fascia of the LD and serratus anterior muscles are included in the flap. When raising the flap, care should be taken to include the underlying fascias of the LD and the anterior serratus muscles. This fascia provides viability to the flap because the flap vascular supply is derived from the lateral intercostal perforators, and the muscular fascia and the lateral perforators from the sixth and seventh intercostal arteries are severed in the dissection. The flap is rotated to the breast defect, and the donor site is closed primarily. The scar of the donor site is located on the patient's back, which can be concealed by the brassiere strap (Figure 3) [11].
This flap provides excellent skin and tissue matching to the native breast, minimal donor site morbidity, and no sacrifice of muscle. However, in patients who have previous surgery to the lateral chest wall, the posterolateral thoracotomy is contraindicated [12].
The upper margin of the flap is drawn at the inframammary fold through a previously designed faint line. Considering the pedicle (based on perforators originating from the superior epigastric artery and veins through the rectus abdominis muscle) of this flap, we draw the lower margin of this flap. A Doppler assessment prior to operation is useful to detect perforators at the presumed flap base [13]. The length of the flap is estimated according to the resection site and the resected volume. The flap consisting of skin and subcutaneous fat is raised from the underlying muscles. In raising the flap around the pedicle, care should be taken to spare the perforators. Between the defect site of the breast and the raised flap, the supramuscular tunnel is created. The portion of the flap that will be positioned under the tunnel is de-epithelized, and the flap is transposed to the defect site through the tunnel. This flap can be used either partially or completely de-epithelized according to the defect site. Due to an abundance of skin and subcutaneous tissue under the breast, the primary suture can usually be performed for the donor site of a flap. The scar can be concealed because it is often located on the inframammary folds. Negative pressure drains are inserted into the donor site, axillary region and breast parenchyma (Figure 4).
This flap can be used to reconstruct defects in the lower quadrant of the breast. By extending the incision of the flap laterally, the upper quadrant of the breast can be reconstructed. If the flap is too long, however, vascular compromise can occur. Patients who have sufficient skin and tissue in the upper abdomen, especially older-aged patients, are well adapted for this procedure. However, this procedure is contraindicated in patients who have a scar as a result of previous surgery in the ipsilateral upper abdomen because of the possibility of the pedicle damage [14].
This flap can be classified according to the pedicles, perforators arising from the costal segment on the lateral aspect of the thorax (lateral ICAP) or perforators from the muscular segment (anterior ICAP) [15]. Regarding the lateral region, the perforators are usually located between 3 and 4.5 cm from the anterior border of the LD muscle (sixth and seventh intercostal spaces). Regarding the medial region, the perforators are usually located within 1-4 cm lateral to the sternal border. Dissection of pedicles is important for tumors especially in the lower quadrants, where a wide dissection below or lateral to the inframammary sulcus may endanger flap vascularization. The incision is carried down to the serratus anterior and LD muscles in the lateral ICAP flap or to the pectoralis muscle or the rectus abdominis muscle in the anterior ICAP flap. The perforators are dissected toward the anterior intercostal (lateral/anterior ICAP) or the internal mammary vessels (anterior ICAP) and are preserved. The skin and the subcutaneous fat are dissected in a subfascial plane. Depending on the defect, the flap is partially or completely de-epithelialized, and the supramuscular tunnel is created between the recipient site and the defect. The flap is folded or tailored to fill the defect. A negative pressure drain is placed, and the donor site is closed primarily (Figure 5).
This flap may be applied as a turnover flap to augment deficiencies of the lower pole of the breast. The flap axis is drawn along the inframammary sulcus so that the resulting scar may be hidden under the brassiere strap. The skin texture of the ICAP flap and the native breast is similar. Moreover, the lateral ICAP flap dissection does not compromise the thoracodorsal vessels [2].
The TDAP flap is supplied by perforating vessels from the thoracodorsal artery, providing the advantage of preserving the LD muscle [16]. The thoracodorsal artery originates from the subscapular artery and descends along the lateral and deep surface of the LD muscle. The thoracodorsal artery provides the serratus branch and perforators, which supply the overlying skin of the lateral chest wall. Thus, a large skin paddle can be designed over these perforators. The flap is elevated at the level of the latissimus fascia. When an appropriate perforating vessel is selected, the dissection is continued through the LD muscle until reaching the thoracodorsal artery and vein. The dissection can be continued superiorly toward the axillary vessels when a longer pedicle and a greater arc of rotation are needed. A tunnel between the anterior edge of the LD muscle and the defect is created through which to pass the flap. The flap is then placed in the defect of the breast (Figure 6) [12].
This flap spares the LD muscle and can reduce the occurrence of postoperative complications at the donor sites, such as restricted shoulder movement and seroma formation, and can thus shorten the recovery period [17].
The LD flap procedure is the first surgical breast reconstruction procedure using pure autologous tissue [18]. However, this procedure cannot provide a sufficient volume of tissue for breast reconstruction in western women who have a relatively large breast size. Thus, the application of this procedure has been diminished recently, compared with the use of a transverse rectus abdominis myocutaneous (TRAM) flap. In Asian women who have small- to moderate-sized breasts, however, this procedure is useful for breast reconstruction following breast-conserving surgery. This flap has a reliable blood supply, but disadvantages include a difficult surgical technique, a prolonged surgical time and postoperative complications at the donor site. One of the most common postoperative complications is seroma formation at the donor site, which occurs with an incidence of approximately 20% [19]. This complication can be markedly minimized by using a quilting suture when suturing the donor site or using fibrin glue [20-22].
A horizontal line is drawn in the dorsal brassiere strap. The available ellipse-shaped transverse skin paddle, which allows for primary closure of the donor site, is marked along this line to conceal the scar. The size of the skin paddle ranges from 6 to 10 cm wide and 20 to 25 cm long. The flap extends horizontally from the posterior axillary fold to the spinous process. The peripheral limits of the extended LD flap are marked on the surface. With patients in the lateral decubitus position, the flap is elevated by raising the adjacent back skin just below the thoracodorsal fascia. It is important to leave a layer of fat to preserve the blood supply to the back skin to avoid wound-healing complications at the donor site. The dissection proceeds inferiorly to the iliac crest and superiorly to the teres major muscle. The vascular branches from the thoracodorsal vessels to the serratus muscle are divided if they interfere with flap rotation, but this is not required in every case. The flap is passed beneath the skin tunnel to the defect. Division of the humeral attachment of the LD muscle helps to increase the arc of rotation of the flap. A negative pressure drain is placed, and the donor site is closed primarily. The patient is placed in the supine position, and the flap is inset (Figure 7) [23].
This flap may be used as a pedicled flap for patients with risk factors for microvascular surgery, such as positive smoking status, diabetes mellitus and obesity. Radiation therapy or chemotherapy following this operation is not contraindicated because this flap is well tolerated. Limiting factors include weakness in the back, shoulder or arm; donor-site morbidity, such as seroma formation; and a large scar on the back (though it can be concealed by the brassiere strap) [24].
In patients with small- to moderate-sized breasts, there is some limitation in acquiring good cosmesis with oncoplastic volume displacement techniques. In smaller breasts, when a postoperative deformity is expected due to a large-volume tumor resection, replacement using non-breast tissue is required. Because breast deformity is common in Korean women after breast-conserving surgery, many of whom have small breasts, oncoplastic volume replacement techniques allow for extensive tumor excision without compromising the cosmetic outcome and can be reliable and useful techniques that provide satisfactory aesthetic results.
Figures and Tables
Figure 1
This figure shows an algorithm of partial breast reconstruction with oncoplastic techniques in small- to moderate-sized breasts.
ICAP=intercostal artery perforator; TDAP=thoracodorsal artery perforator; LD=latissimus dorsi.
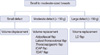
Figure 2
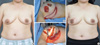
A 43-year-old woman with invasive ductal carcinoma in left lower outer breast. (A) Preoperative view. (B, C) Intraoperative views of elevation and insetting of adipofascial flap after partial mastectomy. (D) 3-month postoperative outcome.
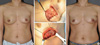
Figure 3
A 51-year-old woman with invasive ductal carcinoma in left upper outer breast. (A) Preoperative view. (B) Intraoperative view of designed lateral thoracodorsal flap after partial mastectomy. (C) Intraoperative view of the elevated flap. (D) 24-month postoperative outcome.
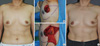
Figure 4
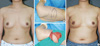
A 60-year-old woman with invasive ductal carcinoma in right upper outer breast. (A) Preoperative view. (B) Intraoperative view of designed thoracoepigastric flap after partial mastectomy. (C) Intraoperative view of the inset flap. (D) 20-month postoperative outcome.
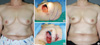
Figure 5
A 39-year-old woman with invasive ductal carcinoma in right upper outer breast. (A) Preoperative view. (B, C) Intraoperative views of elevated intercostal artery perforator flap after partial mastectomy. (D) 13-month postoperative outcome.
Notes
References
1. Yang JD, Bae SG, Chung HY, Cho BC, Park HY, Jung JH. The usefulness of oncoplastic volume displacement techniques in the superiorly located breast cancers for Korean patients with small to moderate-sized breasts. Ann Plast Surg. 2011. 67:474–480.

2. Munhoz AM, Montag E, Arruda E, Brasil JA, Aldrighi JM, Gemperli R, et al. Immediate conservative breast surgery reconstruction with perforator flaps: new challenges in the era of partial mastectomy reconstruction? Breast. 2011. 20:233–240.

3. Yang JD, Lee JW, Kim WW, Jung JH, Park HY. Oncoplastic surgical techniques for personalized breast conserving surgery in breast cancer patient with small to moderate sized breast. J Breast Cancer. 2011. 14:253–261.

4. Losken A, Hamdi M. Partial breast reconstruction: current perspectives. Plast Reconstr Surg. 2009. 124:722–736.

5. Hamdi M, Wolfli J, Van Landuyt K. Partial mastectomy reconstruction. Clin Plast Surg. 2007. 34:51–62.

6. Beahm EK. Nahabedian M, editor. Timing and key considerations in reconstruction for breast-conserving therapy. Oncoplastic Surgery of the Breast. 2009. Philadelphia: Saunders Elsevier;23–46.

7. Kronowitz SJ, Feledy JA, Hunt KK, Kuerer HM, Youssef A, Koutz CA, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg. 2006. 117:1–11.

8. Munhoz AM, Montag E, Arruda E, Aldrighi CM, Filassi JR, Piato JR, et al. Immediate reconstruction following breast-conserving surgery: management of the positive surgical margins and influence on secondary reconstruction. Breast. 2009. 18:47–54.

9. Kijima Y, Yoshinaka H, Funasako Y, Kaneko K, Hirata M, Ishigami S, et al. Immediate reconstruction using thoracodorsal adipofascial flap after partial mastectomy. Breast. 2009. 18:126–129.

10. Kijima Y, Yoshinaka H, Owaki T, Funasako Y, Aikou T. Immediate reconstruction using inframammary adipofascial flap of the anterior rectus sheath after partial mastectomy. Am J Surg. 2007. 193:789–791.

11. Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. Plast Reconstr Surg. 1986. 77:933–943.

12. Levine JL, Reddy PP, Allen RJ. Nahabedian M, editor. Lateral thoracic flaps in breast reconstruction. Oncoplastic Surgery of the Breast. 2009. Philadelphia: Saunders Elsevier;83–92.

13. Uemura T. Superior epigastric artery perforator flap: preliminary report. Plast Reconstr Surg. 2007. 120:1e–5e.

14. Huemer GM. Fitzal F, Schrenk P, editors. Partial mastectomy: breast reconstruction with the pedicled thoracoepigastric flap. Oncoplastic Breast Surgery: A Guide to Clinical Practice. 2010. Vienna: Springer;127–132.
15. Hamdi M, Van Landuyt K, de Frene B, Roche N, Blondeel P, Monstrey S. The versatility of the inter-costal artery perforator (ICAP) flaps. J Plast Reconstr Aesthet Surg. 2006. 59:644–652.

16. Khoobehi K, Allen RJ, Montegut WJ. Thoracodorsal artery perforator flap for reconstruction. South Med J. 1996. 89:Suppl 10. S110.

17. Hamdi M, Salgarello M, Barone-Adesi L, Van Landuyt K. Use of the thoracodorsal artery perforator (TDAP) flap with implant in breast reconstruction. Ann Plast Surg. 2008. 61:143–146.

18. Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980. 65:686–692.

19. Munhoz AM, Montag E, Fels KW, Arruda EG, Sturtz GP, Aldrighi C, et al. Outcome analysis of breast-conservation surgery and immediate latissimus dorsi flap reconstruction in patients with T1 to T2 breast cancer. Plast Reconstr Surg. 2005. 116:741–752.

20. Daltrey I, Thomson H, Hussien M, Krishna K, Rayter Z, Winters ZE. Randomized clinical trial of the effect of quilting latissimus dorsi flap donor site on seroma formation. Br J Surg. 2006. 93:825–830.

21. Weinrach JC, Cronin ED, Smith BK, Collins DR Jr, Cohen BE. Preventing seroma in the latissimus dorsi flap donor site with fibrin sealant. Ann Plast Surg. 2004. 53:12–16.

22. Cho HW, Lew DH, Tark KC. Effect of fibrin sealant in extended lattisimus dorsi flap donor site: retrospective study. J Korean Soc Plast Reconstr Surg. 2008. 35:267–272.
23. Chang DW, Youssef A, Cha S, Reece GP. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002. 110:751–759.

24. Hoch D, Benditte-Klepetko H, Bartsch R, Gösseringer N, Deutinger M. Fitzal F, Schrenk P, editors. Breast reconstruction with the latissimus dorsi muscle flap. Oncoplastic Breast Surgery: A Guide to Clinical Practice. 2010. Vienna: Springer;157–164.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download