Abstract
Purpose
To introduce the history and principle mechanism of electrochemical treatment (EChT) with animal study and report two cases successfully treated breast cancer and hemangioma by EChT.
Methods
In animal study, the breast cancer tumor in nude mouse treated with EChT (100 Coulomb/cm3) were reviewed for histologic changes. In the case studies, we reported method of EChT and clinical results after EChT. Case 1: 74 yr old female with locally advanced breast cancer received 3 times EChT with 1,000 Coulomb/time, 8 Volt. Case 2: 51 yr old female with breast hemagioma received one time EChT with 80 Coulomb, 8 Volt.
Figures and Tables
Fig 1
A case of electrochemical therapy in the back mass of nude mouse. Two platinum electrodes were infiltrated in the tumor.
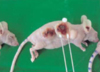
Fig 2
Microscopic findings of breast cancer occurred on the back after inoculation of MDA-MB 231 breast cancer cell-lines to 4th mouse, The section (a: 1:1) is consisted two area. The right side is non-treated tumor (b: ×200) for control and the left side is the tumor after EChT with 80 coulomb (c: ×200). There are destructive change including vaculated cell fragment and necrotic neoplastic cells. An extensive and well-defined area of coagulative necrosis is seen (magnification 10.5X). The anodic lesion is dehydrated, with pycnotic nuclei and a small rim of marginal infarctions at the border (upper area of c). The cathodic lesions are oedematous with cellular swelling and occasional disruption of the plasma membranes (lower area of c). Both anodic and cathodic lesions are divided with very sharp demarcation (black arrow).

Fig 3
Seventyfour years old female has left breast cancer with locally advanced and bone and pleural metastasis. The overlying skin of affected breast shows necrosis and infected area due to advanced breast cancer.
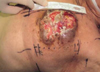
Fig 4
First EChT for the above (Fig 3) patient. After skin sterilization, electrochemical treatments were performed under general anaesthesia at the electrode insertion site. Insertion of electrodes was done under ultrasonogram guidance for proper localization of platinum electrodes. A stylet in a catheter was first inserted into the tumor; then, the stylet was withdrawn. A platinum electrode was inserted through the catheter and was passed into the tumor mass. The catheter was then partially withdrawn in order to treat the tumor and protect the normal tissue from the electricity. After the electrodes were inserted, they were connected to the instrument. Voltage was increased gradually, starting at 2-3 V for a few minutes, then to 4-5 V for a few minutes, and finally to 7-8 V. The final current was usually 40-80 mA. The electricity delivered was calculated at 100 coulombs per cm tumor diameter. Totally thirteen platinum electrodes were used for 1,000 coulombs.
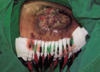
Fig 5
The wound of skin graft shows good quality without local recurrence after thirteen months after skin graft (18 months after 1st EChT).
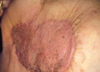
Fig 6
Fiftyone years old female has biopsy confirmed cavernous hemangiomas in left breast and axilla. Preoperative MRI, fat suppression T2 weighted axial image reveals about 1.4 cm sized round high signal intensity nodule in subcutaneous fat layer in left upper outer quadrant. Another nodules also reveals round high signal intensity nodule with round low signal intensity calcification in more upper portion of this lesion.
Fig 7
Two platinum needles are well positioned through two breast nodules in left upper outer quadrant. Position of two platinum needles is well demonstrated on mammography MLO view.

Fig 8
Follow up MRI 2 months later EChT. MRI fat suppression T2 weighted image reveals disappeared previous high signal intensity nodules, but abnormal high signal intensity irregular septae and diffuse skin thickening at EChT site. This finding is suggestive of sclerotic change and fibrosis after EChT.

References
1. Crussel G. Die Electrilytishen Heilanstalt in Moscow. Med. Zeitung Russlands. 1847. 4:2041.
2. Schechter DC. Flashbacks: containment of tumors through electricity. Pacing Clin Electrophysiol. 1979. 2:100–114.

3. Watson BW. The treatment of tumours with direct electric current. Medical Science Research. 1991. 19:103–105.
4. Nordenström BE. Survey of mechanisms in electrochemical treatment (ECT) of cancer. Eur J Surg Suppl. 1994. 574:93–109.
5. Nilsson E, von Euler H, Berendson J, Thörne A, Wersäll P, Näslund I, et al. Electrochemical treatment of tumours. Bioelectrochemistry. 2000. 51:1–11.

6. Humphrey CE, Seal EH. Biophysical approach toward tumor regression in mice. Science. 1959. 130:388–390.

7. Li K, Xin Y, Gu Y, Xu B, Fan D, Ni B. Effects of direct current on dog liver: possible mechanisms for tumor electrochemical treatment. Bioelectromagnetics. 1997. 18:2–7.

8. Nordenström BE. Biologically closed electric circuits: clinical, experimental and theoretical evidence for an additional circulatory system. 1983. Stockholm: Nordic Medical Publications.
9. Nordenström BE. Electrochemical treatment of cancer. I: Variable response to anodic and cathodic fields. Am J Clin Oncol. 1989. 12:530–536.
10. Nordenström BE, Eksborg S, Beving H. Electrochemical treatment of cancer. II: Effect of electrophoretic influence on adriamycin. Am J Clin Oncol. 1990. 13:75–88.
11. Xin YL. Advances in the treatment of malignant tumours by electrochemical therapy (ECT). Eur J Surg Suppl. 1994. 574:31–35.
12. Samuelsson L, Jonsson L. Electrolyte destruction of lung tissue. Electrochemical aspects. Acta Radiol Diagn. 1980. 21:711–714.
13. Samuelsson L, Jonsson L. Electrolytic destruction of tissue in the normal lung of the pig. Acta Radiol Diagn. 1981. 22:9–14.

14. Eksborg S, Nordenstrom BE, Beving H. Electrochemical treatment of cancer. III: Plasma pharmacokinetics of adriamycin after intraneoplastic administration. Am J Clin Oncol. 1990. 13:164–166.
15. Yabushita H, Yoshikawa K, Hirata M, Furuya H, Hojyoh T, Fukatsu H, et al. Effects of electrochemotherapy on CaSki cells derived from a cervical squamous cell carcinoma. Gynecol Oncol. 1997. 65:297–303.

16. Rebersek M, Cufer T, Cemazar M, Kranjc S, Sersa G. Electroche-motherapy with cisplatin of cutaneous tumor lesions in breast cancer. Anticancer Drugs. 2004. 15:593–597.

17. Liu D, Xin YL, Ge B, Zhao F, Zhao H. Experimental studies on electrolytic dosage of ECT for dog's oesophageal injury and clinical effects of ECT for oesophageal anastomotic opening stenosis and oesophageal carcinoma. Eur J Surg Suppl. 1994. 574:71–72.
18. Xin YL. Organisation and spread of electrochemical therapy (ECT) in China. Eur J Surg Suppl. 1994. 574:25–29.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download