Abstract
PURPOSE
The purpose of this study was to investigate the impact of different surface treatment methods and thermal ageing on the bond strength of autopolymerizing acrylic resin to Co-Cr.
MATERIALS AND METHODS
Co-Cr alloy specimens were divided into five groups according to the surface conditioning methods. C: No treatment; SP: flamed with the Silano-Pen device; K: airborne particle abrasion with Al2O3; Co: airborne particle abrasion with silica-coated Al2O3; KSP: flamed with the Silano-Pen device after the group K experimental protocol. Then, autopolymerized acrylic resin was applied to the treated specimen surfaces. All the groups were divided into two subgroups with the thermal cycle and water storage to determine the durability of the bond. The bond strength test was applied in an universal test machine and treated Co-Cr alloys were analyzed by scanning electron microscope (SEM). Two-way analysis of variance (ANOVA) was used to determine the significant differences among surface treatments and thermocycling. Their interactons were followed by a multiple comparison' test performed uing a post hoc Tukey HSD test (α=.05).
Metal substructure of removable partial dentures (RPD) and complete dentures are often fabricated by Co-Cr alloys. Compared to the other alloys, Co-Cr alloys are comparatively economical and have a high rigidity.1,2
Adhesion between denture repair resin and metal framework of a RPD or metal-based complete dentures is essential for the durability of the new prosthesis or for denture repair.2,3,4,5,6 Denture bending while functioning can debond acrylic resin from the framework, and finally fracture the resin.7 In addition, different thermal expansion coefficients of acrylic resin and alloys and the polymerization shrinkage of resin may seperate these materials. Nearly 38% of removable partial denture (RPD) failures include fracture at the alloy-acrylic resin interface.2,7 Microleakage occurs by poor chemical and mechanical (macro or micro) bonding of the alloy-acrylic resin interface and therefore a possibility for adhesive failure can form.5,6
The adhesion of acrylic resin to untreated alloy surfaces is inconsistent.8 Alloy surface treatments have been rated considering the bonding of acrylic resins to alloy. Mechanical bonding depends on macromechanical (loops, mesh, heads, nail, posts, beads bars and struts) or micromechanical (air abrasion, acid etching, electrolytic etching) treatment of alloy framework.7,8,9,10
Micromechanical systems and chemical systems import to reduce the requisite for macromechanical system.8 Micromechanical treatment increases surface area and wettability by enhancing surface-free energy. Apart from air abrasion, most techniques are identified with sensitivity of the technique, time constraints, expensive equipment and hazardous chemicals.11 In addition, chemical bonding between alloy and the acrylic resin is important.6,7 The lack of chemical bonding of a polymethylmethacrylate (PMMA) resin and a metal alloy is an important clinical cause, not seldom causing adhesive failure and increasing microleakage of oral fluids in the finish lines.2,5,6,7 It was indicated that microleakage reduces bond between acrylic resin and metal framework.10 Currently available chemical systems are primer application, tribochemical silane coating (CoJet system, Rocatec), silane coupling (Monobond S), pyrochemical silicoating (Silicoater, Silicoater MD, Silano-Pen), porous metal coating and tin electroplating.6,8,10 Silane treatment is routinely used in the clinic. Chemical reaction occurs with the hydroxyl groups of the silane and the Co-Cr surfaces. Then, it turns into a cross linking structure composed by inter molecular bonding of the methoxy group with autopolymerizing acrylic resin.11 Although these techniques have compared approvingly with micro or macro-mechanical systems, with the exception of primer application, the majority serves as typical of expensive equipment, time constraints and sensitivity of the technique.6,10
Optimal repair methods may obtain a potent adhesive bond. Autopolymerizing acrylic resin is frequently used to repair RPDs and provides for rapid, economical repairs.6,12,13 To increase chemical and micromechanical bond strength of metal framework and resin, many surface treatment methods and different acrylic resin material were evaluated. Sandblasting, silicoating, electro chemically etching, plasma irridation, tinplating, primers (containing 4-META or MDP) are some of these surface treatment.2,4,9,10 The purpose of this study was to investigate impact of different surface treatment methods and thermal ageing on the bond strength of autopolymerizing acrylic resin to Co-Cr alloy. In previous studies many silica-coating techniques (silane coupling, tribochemical silane coating or pyrochemical silica-coating) were evaluated but there is limited report on the comparison of the shear bond strength between autopolymerizing acrylic resin and Co-Cr alloy using Silano-Pen under thermal ageing condition. This study assayed the hypothesis that surface treatments increase the shear bond strength and thermal ageing reduces the repair strength.
Materials used in the study and their compositions are listed in Table 1. Seventy wax patterns in the shape of disc (thickness: 2 mm; diameter: 10 mm) prepared and then Co-Cr alloy (DENTindex CASTCC, Ankara, Turkey) were ordinarily cast by a silica-based investment (Wirovest, BEGO, Bremen, Germany) with centrifugal casting technique. The surfaces of alloys were ground finished using abrasive papers (280-, 400-, 600-, 800- and 1000- grit) (3M ESPE, St. Paul, MN, USA) in the grinding machine under running water for 10 seconds on a 300 rpm (Buehler Metaserv, Buehler, Germany) in order to provide flat and uniform surface and ultrasonically cleaned for 3 minutes with deionized water and dried by air.
All specimens were embedded in the middle of the resin blocks (Meliodent RR, Heraeus Kulzer, Armonk, NY, USA) then all specimens were classified into 5 groups, each containing 14 specimens. One of the groups was control and no surface conditioning was applied (Group C). The specimens in other groups were flamed with the Silano-Pen device (Group SP) for 5 s/cm2, air abraded with 50 µm aluminum oxide (Al2O3) particles (Group K); air abraded with 30 µm silica-coated aluminum oxide particles (Group Co), and air abraded with 50 µm Al2O3 particles and flamed with the Silano-Pen device for 5 s/cm2 (Group KSP). After flaming, specimens were cooled down to room temperature and the Silano-Pen bonding agent (alcoholic solution of 3-methacryloyloxpropytrimethoxy silane) was brushed on and air-dried for 3 minutes. Air abrasion procedure was performed using an intraoral air abrasion device (CoJet System, 3M ESPE, Seefeld, Germany) at a distance of approximately 10 mm for 10 seconds with 2.5 bar pressure. In Group Co, air abrasion was completed with application of a thin silane layer (Espe-Sil, 3M ESPE, Seefeld, Germany), which was left for drying for 5 minutes. After surface treatments, a polytetrafluoroethylene mold (thickness: 2 mm; diameter: 6 mm) was placed on the middle of the specimens and autopolymerized acrylic resin (Meliodent RR, Heraeus Kulzer, Armonk, NY, USA) was applied to specimen surfaces. The specimens were kept for 5 minutes at room temperature then polymerization was completed in a pressure-polymerizing unit (Polyclav, Dentaurum GmbH & Co, Ispringen, Germany) for 30 minutes.
All specimens were stored in distilled water at 37℃ for 24 hours. Each of the groups were divided into two subgroups (n=7) with water storage (TC0) and thermal cycle (TC1) to investigate the durability of the bond. Thermo cycling was performed 6,000 cycles between 5 ± 2℃ and 55 ± 2℃ with a dwell time of 30 seconds in water in every temperature. It was reported that 6,000 thermal cycles would be equivalent to five years clinical use.14
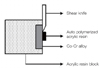
A universal test machine (Lloyd LRX, Lloyd Instruments PIC., Fareham, Hampshire, UK) was used for shear bond strength test at a crosshead speed 0.5 mm/min. Force was applied to the acrylic resin-metal interface (Fig. 1). The calculation of the bond strength values in megapascal (MPa) were obtained by the ratio of the failure load (N) to the area of the acrylic resin (N/πr2).
Five additional specimens were performed with the same experimental protocols as previously mentioned for investigating surface morphology of Co-Cr alloy. They were coated with gold using a sputter coater (S150B; Edwards, Crawley, UK) and examined under a field-emission scanning electron microscope (JSM-6335F, JEOL, Tokyo, Japan) at 20 kV. The SEM photomicrographs were developed at a magnification of ×500 for visual inspection.
To investigate the mode of failure the specimen surfaces were examined visually.
The Kolmogorov-Simirnov test indicated that the data was of a normal distribution (P>.05). Significant differences among surface treatments and thermocycling was shown by two-way analysis of variance (ANOVA)(SPSS 12.0; SPSS Inc., Chicago, IL, USA). Their interactions were followed by a multiple comparisons' test performed using a post hoc Tukey HSD test (α=0.05).
The means and standard deviations (SD) of the repair strengths after 0 and 6,000 cycles, along with the statistical comparisons of the groups are shown in Table 2. Two-way ANOVA indicated that surface treatment, thermo cycling and two-way interactions of surface treatment and thermo cycling were significantly different, and repair strength values varied according to the surface treatments and thermo cycling (P<.001, Table 3). Surface treatments significantly increased repair strengths of repair acrylic resin to Co-Cr alloy. The shear bond strength reduced after thermo cycling. Significant differences were determined between TC0 and TC1 groups except Group C, SP and KSP (P<.001). Specimens treated with Silano-Pen indicated higher bond strength values in thermal cycled conditions (24.13 (SP0), 39.37 (KSP0), 29.00 (KSP1) and 24.02 (SP1), respectively) and significant difference was found between other groups (P<.001), but there was no significant difference between SP0-SP1, and KSP1-KSP2. The shear bond strength was lower in control groups for all thermal aged conditions (3.61 (C1) and 5.93 (C0), respectively) and there was no significant difference between C0 and C1 (P>.05).
SEM photomicrographs of treated Co-Cr alloy surfaces are presented in Fig. 2. SEM photographs showed untreated surface and Silano-Pen treated Co-Cr surface has similar smooth surface topography (Fig. 2A, Fig. 2B). Air abrasion procedure modified the surface by increasing the surface irregularities (Fig. 2C, Fig. 2D). Air abrasion with 50 µm Al2O3 resulted in more irregular and rough surface when compared air abrasion with 30 µm SiOx. Silano-Pen application after air abrasion with 50 µm Al2O3 has some cracks on the rough surface (Fig. 2E).
Visual examination of the fractured surfaces showed that mixed fractures were most associated types of failure for Group SP, K, Co and KSP. Pure acrylic resin cohesive failures were not shown in all groups. The specimens in Group C exhibited 100% adhesive failures. The specimens in Group SP, K, Co and KSP exhibited 64.28%, 64.28%, 71.42% and 78.57% mixed failures, respectively.
The results of the present study accept the research hypotheses that the bond strength of autopolymerized acrylic resin to Co-Cr alloy would be higher after application of surface conditionings.
Higher bond strength values were shown in air abrasion with 50 µm Al2O3 and 30 µm SiOx when compared with the control group. It was previously reported that the bond strength was higher as the bonding surface became rougher.1,2 Air borne particle abrasion creates surface roughness by cleaning the surface of metal-oxides and other substances and increases the mechanical and chemical bond strength between metal and acrylic resin.2,13
A significant decrease of shear bond strength was shown in thermocycling groups. This reduction proposes that adhesive bond between the repair acrylic resin and Co-Cr alloy may be problematic when air abrasion with 50 µm Al2O3 only and 30 µm SiOx were applied and may affect the long term stability of repair strength. According to Na Badalung et al.9 study, which evaluated that bond strength of denture base resins to treated Ni-Cr-Be alloy with different surface treatments, adhesive denture resin groups showed significantly higher bond strength than the traditional base resin and all sandblasted groups showed significantly lower bond strength than the other chemical treated groups. It was not enough to have stronger shear bond strength without chemical retention. It was reported that the mechanical techniques provided lower bond strength values than the chemical and mechanochemical bonding methods.9,15
Sharp et al.10 stated that air abrasion resulted in significant reduce in microleakages when performed alone or in combination with other surface treatment methods (tinplating/oxidation and with silanization). In the present study, application of Silano-Pen increased bond strength. The shear bond strengths of Group C, SP and KSP did not significantly decrease after TC1 (P<.05). The highest bond strength values in TC0 and TC1 were obtained in group KSP that includes air abrasion. In a study Janda et al.,16 higher shear bond strength values with Silano-Pen on titanium, gold and Co-Cr alloy surfaces were obtained. The shear bond strength was decreased in thermocycling except gold alloy.
Silano-Pen is a pyrochemical silica-coating technology. Using a flame treatment approach could perform suitable adhesion. When compared with other pyrochemical silica-coating technologies (Silicoater™ Classic, Silicoater™ MD and Siloc), type of an extra-oral compact hand-piece is used and it can be used not only for the frameworks for RPDs and veneered metallic fixed partial dentures but also for all ceramics.17
It was previously reported that, coating of alloys with the SiOx enhacnes the bond strengths. In this silocoating technique, which obtains the hydroxyl groups required for silane agents bonding to alloy18 a greater resistance to thermocycling was obtained by pyrochemical silicoating. Silicoater method seems to be a reliable pretreatment of the metal.
Increased bond strength of resin to Ni-Cr-Be alloy was found with silica-coating and etching with hydrogen fluoride, hydrogen chloride and nitric acid.9 In another study, heat polymerized denture base resin exhibited higher bond strength values to sandblasted and silicoated titanium alloy specimens than sandblasted titanium alloy specimens alone.7
Ohkubo et al.1 evaluated that bond strength of PMMA resin to metal frameworks applied by five metal primers. The result of the study showed that performing of five primers significantly increased the bond strength. Siloc bonding system (Heraeus Kulzer, Ivrine, CA, USA) has been improved to achieve a chemical-micromechanical bond between PMMA and alloys. Besides, this method supposes not only the performing of a coating, but also heat treatment to activate the coating agent. In the present study, Silano-Pen groups showed higher bond strength values when compared to other surface treatments (polishing, air abrasion only).
Shimizu et al.5 evaluated that use of various metal primers to improve bond strengths of autopolymerizing denture base resin to cast alloys used for RPDs. They reported that metal conditioners improved bond strengths but after thermocycling shear bond strength decreased significantly.
Kim et al.3 evaluated that the effect of ultrasonic, steam, boiling and thermal cycling on shear bond strength of veneer resin on a chemically pretreated Co-Cr metal alloy and denture teeth. The result of that study showed that laboratory procedures and thermal cycling caused a significant reduction in bond strength.
Ishii et al.15 examined the impact of air-borne particle abrasion with alumina with varied pressure on bonding acrylic resin to different alloys. The unabraded specimens exhibited the lowest strength in the study. Thermocycling resulted in decreased shear bond strength but air-borne particle abrasion with alumina with an air pressure of 0.6 MPa is efficient in improving the retention of the acrylic resin connected to the alloys.14
The shear bond strength is not the only factor that may affect the stability of resin-metal bonds. The current study was limited in the simulation of the intraoral situation as dynamic fatigue loading and pH changes. The efficacy of the performed systems in providing suitable bond strength should be confirmed by other researchs, including long-term clinical studies.
According to the result of the present study, thermocycling caused a significant decrease in adhesion for application of a thin silane layer after air abrasion with 30 µm silica-coated aluminum oxide particles. On the contrary, applying Silano-Pen bonding agent after flaming with Silano-Pen did not show a significant decrease in adhesion after termocycling. This result can be ascribed as the impact of heat treatment on the prolonged stability of bond strength.
Figures and Tables
Fig. 2
SEM photographs of the Co-Cr specimens. (A) control, (B) flamed with Silano-Pen, (C) air abraded with 50 µm Al2O3, (D) air abraded with 30 µm SiOx, (E) air abraded with 50 µm Al2O3and flamed with Silano-Pen (white arrows indicate cracks).

Table 1
Materials used in the study

Table 2
Mean repair strength (MPa) and standard deviations (SD)

References
1. Ohkubo C, Watanabe I, Hosoi T, Okabe T. Shear bond strengths of polymethyl methacrylate to cast titanium and cobalt-chromium frameworks using five metal primers. J Prosthet Dent. 2000; 83:50–57.
2. Bulbul M, Kesim B. The effect of primers on shear bond strength of acrylic resins to different types of metals. J Prosthet Dent. 2010; 103:303–308.
3. Kim JY, Pfeiffer P, Niedermeier W. Effect of laboratory procedures and thermocycling on the shear bond strength of resin-metal bonding systems. J Prosthet Dent. 2003; 90:184–189.
4. Maruo Y, Nishigawa G, Oka M, Minagi S, Suzuki K, Irie M. Does plasma irradiation improve shear bond strength of acrylic resin to cobalt-chromium alloy? Dent Mater. 2004; 20:509–512.
5. Shimizu H, Kurtz KS, Tachii Y, Takahashi Y. Use of metal conditioners to improve bond strengths of autopolymerizing denture base resin to cast Ti-6Al-7Nb and Co-Cr. J Dent. 2006; 34:117–122.
6. Banerjee S, Engelmeier RL, O'Keefe KL, Powers JM. In vitro tensile bond strength of denture repair acrylic resins to primed base metal alloys using two different processing techniques. J Prosthodont. 2009; 18:676–683.
7. Pesun S, Mazurat RD. Bond strength of acrylic resin to cobalt-chromium alloy treated with the Silicoater MD and Kevloc systems. J Can Dent Assoc. 1998; 64:798–802.
8. Mudford L, Curtis RV, Walter JD. An investigation of debonding between heat-cured PMMA and titanium alloy (Ti-6A1-4V). J Dent. 1997; 25:415–421.
9. NaBadalung DP, Powers JM, Connelly ME. Comparison of bond strengths of denture base resins to nickel-chromium-beryllium removable partial denture alloy. J Prosthet Dent. 1997; 78:566–573.
10. Sharp B, Morton D, Clark AE. Effectiveness of metal surface treatments in controlling microleakage of the acrylic resin-metal framework interface. J Prosthet Dent. 2000; 84:617–622.
11. Sanohkan S, Urapepon S, Harnirattisai C, Sirisinha C, Sunintaboon P. Shear bond strength between autopolymerizing acrylic resin and Co-Cr alloy using different primers. Dent Mater J. 2012; 31:765–771.
12. Rached RN, Powers JM, Del Bel Cury AA. Repair strength of autopolymerizing, microwave, and conventional heat-polymerized acrylic resins. J Prosthet Dent. 2004; 92:79–82.
13. Nagai E, Otani K, Satoh Y, Suzuki S. Repair of denture base resin using woven metal and glass fiber: effect of methylene chloride pretreatment. J Prosthet Dent. 2001; 85:496–500.
14. Leibrock A, Degenhart M, Behr M, Rosentritt M, Handel G. In vitro study of the effect of thermo- and load-cycling on the bond strength of porcelain repair systems. J Oral Rehabil. 1999; 26:130–137.
15. Ishii T, Koizumi H, Tanoue N, Naito K, Yamashita M, Matsumura H. Effect of alumina air-abrasion on mechanical bonding between an acrylic resin and casting alloys. J Oral Sci. 2009; 51:161–166.
16. Janda R, Roulet JF, Latta M, Damerau G. Spark erosion as a metal-resin bonding system. Dent Mater. 2007; 23:193–197.
17. Matinlinna JP, Vallittu PK. Silane based concepts on bonding resin composite to metals. J Contemp Dent Pract. 2007; 8:1–8.
18. Clelland NL, van Putten MC, Brantley WA, Knobloch LA. Adhesion testing of a denture base resin with 5 casting alloys. J Prosthodont. 2000; 9:30–36.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download