Abstract
Somatic mutations that lead to hyperactivation of epidermal growth factor receptor (EGFR) signaling are detected in approximately 50% of lung adenocarcinoma in people from the Far East population and tyrosine kinase inhibitors are now the standard first line treatment for advanced disease. They have led to a doubling of progression-free survival and an increase in overall survival by more than 2 years. However, emergence of resistant clones has become the primary cause for treatment failure, and has created a new challenge in the daily management of patients with EGFR mutations. Identification of mechanisms leading to inhibitor resistance has led to new therapeutic modalities, some of which have now been adapted for patients with unsuccessful tyrosine kinase inhibitor treatment. In this review, we describe mechanisms of tyrosine kinase inhibitor resistance and the available strategies to overcoming resistance.
Lung cancer remains a major cause of cancer death worldwide. In the year 2012, 1,824,701 new lung cancer cases were diagnosed, and 1,590,000 patients died of this devastating disease worldwide1. Non-small cell lung cancer (NSCLC) comprises 80%–85% of lung cancers and more than half of NSCLC cases are discovered at an advanced stage, which requires systemic treatment. Among the changes of the pathologic subtypes of lung cancer, the proportion of adenocarcinoma, which has higher instances of driver mutations, is gradually increasing.
Epidermal growth factor receptor (EGFR, ErbB1, HER1) is a cell surface receptor from the ErbB receptor family and is one of four related receptor tyrosine kinases, along with HER2/c-neu (ErbB2), HER3 (ErbB3), and HER4 (ErbB4)2. After ligand binding, EGFR changes from an inactive monomer to an active homodimer. It may also form a heterodimer with another member of the ErbB receptor family. EGFR dimerization induces intrinsic protein-tyrosine kinase activity that results in autophosphorylation of its C-terminal tyrosine residues34. Autophosphorylation of EGFR activates downstream signaling cascades such as the AKT, mitogen-activated protein kinase (MAPK), and JNK pathways, which ultimately leads to DNA synthesis, cell cycle progression, and proliferation5.
Mutation, amplification, or dysregulation of the EGFR family is proposed to occur in approximately 30% of epithelial cancers. Somatic mutations that lead to EGFR hyperactivation are associated with a number of cancers, including NSCLC, colorectal cancer, and glioblastoma multiforme67. These somatic mutations involving the EGFR tyrosine kinase domain (TKD) lead to the persistent activation of its downstream signaling pathways, which results in uncontrolled cell proliferation8.
EGFR tyrosine kinase inhibitors (TKI) have been widely described as being useful for the treatment of lung cancer patients with EGFR-TKI–sensitizing mutations, and their use has led to a doubling of progression-free survival910111213 and a lengthening of overall survival by more than 2 years. However, emergence of resistant cancers has become the ultimate reason for treatment failure, which creates new challenges for the daily management of patients with EGFR mutations14. In this review, we describe the mechanism behind EGFR-TKI resistance and the strategies available for overcoming resistance to EGFR-TKI.
It is well established that 50%–80% of NSCLC overexpress EGFR. Consequently, there have been numerous treatments developed using antibodies and small molecules that inhibits EGFR. The first generation EGFR-specific inhibitors, gefitinib (ZD1839, Iressa) and erlotinib (Tarceva), inhibit EGFR activity by reversibly binding to the adenosine triphosphate (ATP)-binding site in the TKD (Table 1). In the early 2000s, the IDEAL I/II study, which recruited previously treated NSCLC patients, was performed to compare the efficacy and adverse effects between the 250 mg and 500 mg treatments of gefitinib. The researchers found that daily administration of 250 mg of gefitinib had an 18.4% response rate with a 95% confidence interval, 11.5–27.3. The difference in the response rate between 250 mg and 500 mg was not significant. However, the 250 mg treatment group had a lower frequency of side effects, and so the U.S. Food and Drug Administration (U.S. FDA) approved gefitinib 250 mg daily for the treatment of NSCLC15. The subsequent phase III study, ISEL, which compared placebo and gefitinib treatment groups in previously treated NSCLC patients, failed to demonstrate any survival benefits in the gefitinib treatment group. This led to the discontinuation of gefitinib use in the United States16. However, in 2004, mutations in the EGFR-TKD were identified and some of the patients who had mutations in EGFR-TKD showed an excellent response to the EGFR-TKI817. The L858R point mutation in exon 21 and deletion mutations in exon 19 that makes up the leucine-arginine-glutamine-alanine (LREA) motif (amino acids 747 to 750) were identified as key sensitizing mutations that predict a positive response to EGFR-TKI. Following these trials, patients with EGFR mutations were recruited for further studies, and a response rate of 60%–80%, along with a progression-free survival of approximately 10 months1819. These results helped to establish that specific genetic mutations could predict response rate, progression-free survival, and survival rate following drug treatment, and they highlight the importance of predictive biomarkers for drug response.
Acquired resistance to the first generation EGFR-TKI had accelerated the clinical development of second generation EGFR-TKI, which is characterized by irreversible covalent attachments to the EGFR-TKD and additional activity against other receptors which belong to EGFR family or structurally similar receptors20. Several drugs with these characteristics had started clinical trials but most of the second generation EGFR-TKI, except afatinib, had failed because of lack of clinical efficacy or limitation of clinical dosing due to toxicity in NSCLC.
Activating EGFR mutations are frequently identified in lung adenocarcinoma, and advances in genotyping technology has facilitated the discovery of such mutations in small size samples2122. The most frequently detected type of EGFR mutation is an in-frame deletion of exon 19 (E19del) near the LREA motif that accounts for approximately 45% of mutations in the TKD, as well as the L858R point mutation of exon 21 that accounts for approximately 40% of TKD mutations232425. In addition to E19del and L858R mutations, less frequent mutations, such as point mutations in exon 18 at position G719 (approximately 3% occurrence rate) and the exon 21 L861Q mutant (approximately 2%), are sensitive to EGFR-TKI262728. On the other hand, an in-frame insertion within exon 20 of EGFR, which accounts for 4%–10% of all TKD mutations, and other rare mutations including L747S, D761Y, T790M, and T854A confer resistance to EGFR-TKI293031.
As more sensitive and precise tumor genotyping systems have been made available for clinical applications, rare EGFR mutations of unknown biological and clinical significance are frequently encountered in routine practice22. Different responses to EGFR-TKI have been reported for mutations within the same approximate genomic location. For example, in-frame insertions within EGFR exon 20 were originally considered to confer EGFR-TKI resistance with a low response rate (5%) and short interval of disease control; however, A763_Y764insFQEA has been reported to be a sensitizing mutation to EGFR-TKI31. These findings indicate that more attention and collaborative efforts are required to elucidate the biological and clinical significance of these rare and diverse EGFR mutations.
Even if EGFR-TKI is administered after confirmation of a mutation in the EGFR-TKD, some patients do not respond to EGFR-TKI at all (de novo/intrinsic resistance), and the remaining patients who had a favorable response at initial treatment eventually exhibit disease progression after 10–14 months (acquired resistance). Several mechanisms regulating resistance have been identified, and they may be classified as intrinsic and acquired resistance (Figure 1)32.
BCL2-like 11 (BIM) is a pro-apoptotic molecule of the BCL2 family and is required for TKI-induced apoptotic cell death. The intron 2 deletion polymorphism of BIM causes a splicing switch between exon 4 and exon 3, resulting in a BIM isoform variant lacking the pro-apoptotic BCL2-homology 3 (BH3) domain. The BH3 domain interacts with other members of the BCL2 protein family, including BCL2, BCL2L1/BCL-X(L), and MCL1 and inhibits their anti-apoptotic function. The intron 2 deletion polymorphism confers intrinsic TKI resistance for NSCLC patients with EGFR mutations, resulting in significantly inferior responses33. The BIM intron 2 deletion polymorphism is rare in Western populations but is found in about 12%–15% of Far East Asian populations. This polymorphism does not increase the risk of lung cancer; instead, it limits the response to EGFR-TKI3334.
Not only have advances in sequencing technology enabled researchers to acquire more accurate data, but they have also provided a higher probability of identifying a greater number of mutations in the EGFR-TKD from smaller samples. Compound EGFR mutations, defined as double or multiple mutations in the EGFR-TKD, are frequently detected with newer sequencing technology. A study analyzing 61 cases of EGFR mutation-positive lung adenocarcinoma with next-generation sequencing (NGS)–based repeated deep sequencing found that compound EGFR mutations account for 24.6% of EGFR mutation-positive lung adenocarcinoma. The majority of compound EGFR mutations are a combination of atypical mutations and typical mutations, such as E19del and L858R2235. Kobayashi et al.36 showed that compound EGFR mutations in which an EGFR-TKI–sensitizing mutation—such as G719X, E19del, L858R, or L861Q—coexist with rare mutations of other residues of the TKD that are somewhat sensitive to EGFR-TKI. Peng et al.37 compared the clinical outcome between NSCLC patients with L858R single mutation with patients harboring both a L858R mutation and a co-mutation in EGFR exon 18–21 and found no significant differences in overall survival and progression-free survival. Another study addressed the clinical significance of compound EGFR mutations and found that patients with rare atypical mutations and either E19del or L858R had a less favorable outcome compared with patients who have a single typical mutation. Compound mutations that contain sensitizing mutations, such as G719X or L858R, seem to have favorable responses to EGFR-TKI. On the other hand, compound mutations consisting of rare atypical mutations have a poor response to EGFR-TKI35. Taken together, the patients with compound EGFR mutations have a shorter overall survival than those with simple mutations and, therefore, they should be closely monitored during follow-up.
The concept that any given cancer will have a single driver mutation is now being challenged by data acquired from sequencing multiple genes simultaneously. Although EGFR mutations are the only driver mutation in many cases of lung adenocarcinoma, driver mutations in other genes are found intermittently with advances in sequencing technology. When the EGFR mutation status was reassessed with target enriched NGS in anaplastic lymphoma kinase (ALK) rearrangement-positive NSCLCs, the co-mutation rate was found to be as high as 15.4%. In this study, authors evaluated the response to TKIs in 14 NSCLC patients with EGFR and ALK co-alteration. Among them, three were treated with EGFR-TKI and had poor responses to gefitinib, whereas the eight treated with ALK inhibitors had favorable responses, suggesting that signaling from ALK rearrangement may override EGFR38. Another study found that mutations in exon 9 and 20 of the PIK3CA gene occurred in 3.9% of squamous cell cancers and 2.7% of adenocarcinomas. Among 34 NSCLC cases harboring PIK3CA mutations, 17 cases had a co-mutation in EGFR exon 18–21 and 4 cases had a co-mutation in KRAS exon 2–3, indicating that PIK3CA mutations are frequently accompanied by EGFR/KRAS mutations. These studies revealed the importance of identifying PIK3CA mutations by demonstrating that patients with PIK3CA single mutation have a poorer prognosis than those with co-mutation of PIK3CA and EGFR/KRAS39.
The T790M gatekeeper mutation, localized in exon 20 of EGFR, has been found for the first time to confer resistance to EGFR-TKI40 and can be identified in about half of patients who experience resistance after treatment with first generation EGFR-TKI41. This threonine-to-methionine substitution leads to an increased affinity of EGFR to ATP compared with its affinity to first generation of EGFR-TKI. The bulkier methionine residue at position 790 sterically inhibits the interaction with the inhibitor, effectively preventing its binding to the TKD while preserving catalytic activity. The T790M mutation has been rarely identified in the EGFR-TKI naïve patients in Western countries42. A few EGFR-TKI–resistant mutations have also been identified, such as T854A in exon 21, D761Y, and L747S, but their clinical relevance remains unclear434445.
Three drugs have been developed for patients with the T790M mutation and are ready for clinical application: CO-1686 (Clovis, Rociletinib), which was first developed specifically for T790M; AZD9291 (AstraZeneca, Tagrisso, Osimertinib); and BI1482694/HM61713 (Hanmi, Olita, Olmutinib). Third generation EGFR mutant selective inhibitors (EMSIs) are characterized by sparing wild type EGFR, selective inhibition of T790M, and irreversible covalent binding to target. A brief comparison is available in Table 146. CO-1686 demonstrated its efficacy in treating T790M-positive NSCLC during TIGER-X (Phase 2), TIGER-1 (Phase 2/3), TIGER-2 (Phase 2), and TIGER-3 (Phase 3) studies; however, hyperlipidemia, nausea, vomiting, and corrected QT interval prolongation became primary concerns. AZD9291 also demonstrated its efficacy in treating T790M-positive patients through AURA1, AURA2, and FLAURA trails. Although it had less severe adverse effects than gefitinib, there were reports of skin rashes, diarrhea, and, in some cases, interstitial pneumonitis. AZD9291 was first approved by the U.S. FDA for the treatment of EGFR mutation–positive NSCLC among the third generation EMSI and is now available in our medical fields. ASP8273 (Astellas) and EGF816 (Novartis) were recently developed; however, resistance against these third generation EGFR-TKI eventually develop, possibly through the C797S mutation.
MET amplification was first identified in EGFR-TKI–sensitive lung cancer cells that develop resistance after prolonged exposure to gefitinib as a result of focal amplification of the MET gene. MET amplification was identified in four of 18 lung cancers that had developed resistance to EGFR-TKI through ErbB3-dependent activation of phosphoinositide 3-kinase (PI3K)47. Although the frequency of MET amplification in treating naïve NSCLC is rare, MET amplification is the second most commonly acquired resistance mechanism, occurring up to 20% during EGFR-TKI treatment. Cell lines with MET gene amplification are extremely sensitive to MET inhibition, and the majority of preclinical and clinical data demonstrate that co-inhibition of MET and EGFR may be an effective strategy to overcoming resistance. A trial involving use of the MET inhibitor onartuzumab in combination with erlotinib in heavily treated patients had promising results: MET-positive patients assessed by immunohistochemistry had increased progression-free survival and overall survival compared with MET-negative patients48. Following the METLung phase III trial, which compared the combination of onartuzumab and erlotinib with erlotinib alone in MET-positive and EGFR mutation-positive patients did not show any differences between the groups at interim analysis, and so the trial was terminated early. Ficlatuzumab is an anti-MET monoclonal antibody that targets the hepatocyte growth factor. In a phase II trial recruiting EGFR sensitizing mutation-positive Asian patients, the combination of ficlatuzumab and gefitinib did not improve response rate or progression-free survival. However, a subset of patients with EGFR sensitizing mutation positive and MET-low expression patients benefited from this combination, suggesting ficlatuzumab may inhibit emergence of resistant clones49. The MARQUEE trial (MET inhibitor ARQ 197 plus erlotinib versus erlotinib plus placebo in NSCLC) and ATTENTION trial (Asian trial of tivantinib plus erlotinib versus erlotinib for NSCLC without EGFR mutation) were both phase III trials that evaluated tivantinib, a selective MET inhibitor used previously to treat unselected patients. Both studies did not reveal a difference in overall survival. Cabozantinib (XL184/BMS-907351; Exelixis/Bristol Myer Squibb), foretinib (XL880, EXEL-2880, GSK1363089; Exelixis/GSK), MetMAb (Genentech-Roche), and crizotinib (PF-2341066; Pfizer) have been developed as MET inhibitors and are undergoing clinical trials50. Although MET poses as an ideal biological target whose amplification is frequently observed in EGFR-TKI treated patients, its clinical benefit is still modest.
HER2 amplification or overexpression is associated with more aggressive types of cancer. HER2 amplification is a rare event that occurs in 1%–2% of untreated lung adenocarcinoma as measured by fluorescence in situ hybridization (FISH), a microarray-based genomic copy number analysis. A study that adapted FISH to measure HER2 amplification found that 12% of tumors had acquired resistance to EGFR-TKI, and that this event is mutually exclusive to those with the T790M mutation51. Because afatinib is a second generation irreversible pan-HER inhibitor, it was expected to be effective in the patients previously treated with first generation EGFR-TKI. LUX-Lung 4 trial, a phase II single-arm study, enrolled 62 patients that were previously treated with gefitinib and/or erlotinib and also with afatinib. Although not all the patients in this study harbored HER2 amplification, the effect of afatinib was modest and had an overall response rate of 8.2%, and progression-free survival was found to be 4.4 months52. Based on these findings, Takezawa et al.51 suggested that the combination of afatinib and cetuximab would be beneficial for patients without the T790M mutation who experienced acquired resistance after EGFR-TKI treatment. The combination of afatinib and cetuximab induces tumor regression by decreasing the phosphorylation of EGFR and total EGFR expression, resulting in inhibition of its signaling pathways53. In a phase Ib trial that combined cetuximab and afatinib for 126 NSCLC patients whose disease progressed during EGFR-TKI treatment, the overall response rate was 29% and progression-free survival was 4.7 months. Interestingly, this combination was equally effective in T790M-positive and negative patients54, implying that the efficacy of the combination is attribute to the intrinsic effect of afatinib or associated with any EGFR-TKI combined with cetuximab needs to be clarified.
PIK3CA, which encodes the catalytic subunit of PI3K, is occasionally mutated in lung cancer. Mutations usually occur in its helical domain (exon 9) or kinase domain (exon 20), and the E542K, E545K, E545Q, H1047L, and H1047R mutations have been identified as activating mutations of PIK3CA32. In addition, PIK3CA mutations have been detected in a small percentage (approximately 5%) of EGFR-mutated lung cancers with acquired resistance to EGFR-TKI therapy. Downstream mutations in PIK3CA are rare, but have been identified as a mechanism of resistance55. Recently, a study demonstrated that PIK3CA mutations frequently coexist with EGFR or KRAS mutations56. NSCLC patients with PIK3CA mutation have a poor prognosis. The role of mutant PIK3CA in oncogenic signaling requires further investigation, including development of novel targets for therapeutic intervention in cancers harboring PIK3CA mutations.
BRAF mutations are commonly detected in malignant melanoma: 30%–40% of cases have an activating mutation, whereas it is only detected in approximately 1% of NSCLC. The G469A, V600E, and V599E mutations in the BRAF gene are found in a number of tumors, including malignant melanoma, colorectal, and lung cancer. V599E is one of the common activating mutations and causes constitutive activation of the RAF/MEKL/ERK signaling cascade, resulting in pro-mitogenic activity and decreased drug response57. Vemurafenib and dabrafenib was found to be effective in treating malignant melanoma and are now under clinical evaluation for treating lung adenocarcinoma.
AXL belongs to the Tyro/AXL/Mer (TAM) receptor tyrosine kinase family and is involved in cell survival, growth, and metastasis5859. Recently, several reports have indicated that the aberrantly activated AXL could contribute to the development of acquired resistance to targeted agents such as imatinib, lapatinib, and erlotinib555660. According to the combined work of three independent research groups60, in vivo xenograft and in vitro cell line cancer models with AXL-mediated resistance were established by the continued treatment with erlotinib. The genetic and chemical inhibition of AXL was able to restore sensitivity to erlotinib in these models. Furthermore, re-biopsy samples acquired from patients with EGFR-TKI resistance were found to have increased expression of AXL as assessed by an immunohistochemistry analysis. Although this evidence is quite compelling, there remain several concerns, such as how accurately an immunohistochemistry analysis of AXL expression can reflect the bypassing of AXL signal activation, which is associated with resistance. In addition, further study is needed to determine the exact proportion of patients that could benefit from the suppression of AXL signaling. Although some multi-kinase inhibitors with the ability to inhibit AXL and several specific AXL inhibitors are under development61, there are currently no potent selective AXL inhibitors available that can be used in clinical trials, which makes it difficult to confirm the exact role of AXL in acquired resistance to EGFR-TKI.
Transformation of small cell lung cancer has been seldom observed after the development of drug resistance. Tumor cells with the small cell lung cancer phenotype that retain their original EGFR mutations are effectively treated with standard chemotherapy; however, after a period of conventional cytotoxic chemotherapy, these tumors become sensitized to EGFR-TKI. The mechanism underlying this phenomenon is still unknown.
Epithelial-mesenchymal transition (EMT) is a regular physiological process that occurs during embryogenesis, wound healing, and tissue regeneration; however, it is also observed in a few pathological conditions. EMT is characterized by the loss of E-cadherin and the gain of mesenchymal markers, such as vimentin and N-cadherin32. Through EMT, cancer cells gain mesenchymal properties and become more motile and invasive. Although the specific mechanism of EMT remains unclear, there may be several mechanisms at play during EMT. Activation of AXL by the PI3K/AKT pathway and loss of E-cadherin by activation of EGFR-MEK-ZEB1-MMP2 axis may induce EMT, thereby promoting cancer cell invasion and progression. Other EMT-inducing signals include those initiated by transforming growth factor β, fibroblast growth factor, epidermal growth factor, and hepatocyte growth factor, and pathways or factors associated with EMT include Notch, interleukin 6, SOX9, FoxO4, and SRC.
Through the identification of resistance mechanisms during EGFR-TKI treatment, new therapeutic modalities have been adapted for patients with unsuccessful treatments. These adaptations have had favorable results in some cases. Among them, the third generation EMSIs had outstanding therapeutic effects, and some of these drugs are now available for use in the medical field. On the other hand, inhibition of alternative pathways—such as MET amplification, HER2 amplification, PI3K/AKT/mammalian target of rapamycin signaling, and MAPK signaling—is theoretically sound, and many TKIs have been developed. However, their clinical benefit remains modest,
and further clinical evaluation is required. Further evaluation
of their biologic impact and careful selection of patients that would benefit from these TKIs may result in effective treatment of patients who were not successfully treated with first generation EGFR-TKI.
Figures and Tables
Figure 1
Resistance mechanism against first generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI). (A) Mutations in the EGFR gene. T790M mutation induces conformational changes on the ATP-binding pocket of EGFR–tyrosine kinase domain, inhibiting interaction with the drug target site. (B) Activation of alternative signaling pathways. MET amplification, and overexpression of phosphoinositide 3-kinase (PI3K)/AKT, mitogen-activated protein kinase (MAPK), and AXL bypass the dependency on EGFR activation and can promote survival and proliferation. (C) Phenotypic changes, small cell lung cancer transformation, and epithelial-mesenchymal transition (EMT) confer resistance to EGFR-TKI. SCLC: small cell lung cancer.
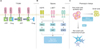
Table 1
EGFR-TKIs currently in use or under development

References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
2. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004; 59:2 Suppl. 21–26.
3. Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987; 26:1443–1451.
4. Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984; 311:483–485.
5. Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005; 1:2005.0010.
6. Walker F, Abramowitz L, Benabderrahmane D, Duval X, Descatoire V, Henin D, et al. Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum Pathol. 2009; 40:1517–1527.
7. Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001; 8:83–96.
8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139.
9. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957.
10. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388.
11. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128.
12. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246.
13. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.
14. Zhou C, Yao LD. Strategies to improve outcomes of patients with EGRF-mutant non-small cell lung cancer: review of the literature. J Thorac Oncol. 2016; 11:174–186.
15. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003; 21:2237–2246.
16. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–1537.
17. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004; 101:13306–13311.
18. Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009; 27:1394–1400.
19. Sequist LV, Martins RG, Spigel D, Grunberg SM, Spira A, Janne PA, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008; 26:2442–2449.
20. Yu HA, Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in lung cancers. J Natl Compr Canc Netw. 2013; 11:161–169.
21. Kim HS, Sung JS, Yang SJ, Kwon NJ, Jin L, Kim ST, et al. Predictive efficacy of low burden EGFR mutation detected by next-generation sequencing on response to EGFR tyrosine kinase inhibitors in non-small-cell lung carcinoma. PLoS One. 2013; 8:e81975.
22. Kim EY, Cho EN, Park HS, Hong JY, Lim S, Youn JP, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther. 2016; 17:237–245.
23. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005; 97:339–346.
24. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007; 25:587–595.
25. Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005; 11:1167–1173.
26. Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010; 277:301–308.
27. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007; 98:1817–1824.
28. Yeh P, Chen H, Andrews J, Naser R, Pao W, Horn L. DNA-mutation Inventory to Refine and Enhance Cancer Treatment (DIRECT): a catalog of clinically relevant cancer mutations to enable genome-directed anticancer therapy. Clin Cancer Res. 2013; 19:1894–1901.
29. Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010; 10:760–774.
30. Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012; 13:e23–e31.
31. Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013; 5:216ra177.
32. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011; 3:75ra26.
33. Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012; 18:521–528.
34. Cho EN, Kim EY, Jung JY, Kim A, Oh IJ, Kim YC, et al. BCL2-like 11 intron 2 deletion polymorphism is not associated with non-small cell lung cancer risk and prognosis. Lung Cancer. 2015; 90:106–110.
35. Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011; 17:3812–3821.
36. Kobayashi S, Canepa HM, Bailey AS, Nakayama S, Yamaguchi N, Goldstein MA, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013; 8:45–51.
37. Peng L, Song Z, Jiao S. Comparison of uncommon EGFR exon 21 L858R compound mutations with single mutation. Onco Targets Ther. 2015; 8:905–910.
38. Won JK, Keam B, Koh J, Cho HJ, Jeon YK, Kim TM, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015; 26:348–354.
39. Wang L, Hu H, Pan Y, Wang R, Li Y, Shen L, et al. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One. 2014; 9:e88291.
40. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005; 352:786–792.
41. Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006; 12:6494–6501.
42. Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012; 30:433–440.
43. Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001; 293:876–880.
44. Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002; 2:117–125.
45. O'Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005; 65:4500–4505.
46. Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013; 3:1404–1415.
47. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–1043.
48. Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013; 31:4105–4114.
49. Mok TS, Park K, Geater SL, Agarwal S, Han M, Komarnitsky P, et al. A randomized phase 2 study with exploratory biomarker analysis of ficlatuzumab a humanized hepatocyte growth factor (HGF) inhibitory monoclonal antivody, in combination with gefitinib verus gefitinib in Asian patients with lung adenocarcinoma. Ann Oncol. 2012; 23:Suppl 9. 1198P.
50. Feng Y, Thiagarajan PS, Ma PC. MET signaling: novel targeted inhibition and its clinical development in lung cancer. J Thorac Oncol. 2012; 7:459–467.
51. Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012; 2:922–933.
52. Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol. 2013; 31:3335–3341.
53. Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009; 119:3000–3010.
54. Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014; 4:1036–1045.
55. Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009; 69:6871–6878.
56. Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007; 26:3909–3919.
57. Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012; 109:E2127–E2133.
58. Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008; 100:35–83.
59. Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006; 17:295–304.
60. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012; 44:852–860.
61. Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK, Yoon SJ, et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014; 74:253–262.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download