Abstract
Active tuberculosis (TB) has a greater burden of TB bacilli than latent TB and acts as an infection source for contacts. Latent tuberculosis infection (LTBI) is the state in which humans are infected with Mycobacterium tuberculosis without any clinical symptoms, radiological abnormality, or microbiological evidence. TB is transmissible by respiratory droplet nucleus of 1–5 µm in diameter, containing 1–10 TB bacilli. TB transmission is affected by the strength of the infectious source, infectiousness of TB bacilli, immunoresistance of the host, environmental stresses, and biosocial factors. Infection controls to reduce TB transmission consist of managerial activities, administrative control, engineering control, environmental control, and personal protective equipment provision. However, diagnosis and treatment for LTBI as a national TB control program is an important strategy on the precondition that active TB is not missed. Therefore, more concrete evidences for LTBI management based on clinical and public perspectives are needed.
Tuberculosis (TB) is an infectious disease that has existed throughout human history. However, Mycobacterium tuberculosis was confirmed as the causative pathogen by Robert Koch only in the early 19th century, and the airborne nature of TB infection was proved objectively even later, in the 1950s. TB develops in only 10% of humans exposed to M. tuberculosis. Moreover, TB generally develops within 1–2 years of M. tuberculosis infection in 5% of those infected, and at any other time in the remaining 5%1. Latent tuberculosis infection (LTBI) is the state in which humans are infected with M. tuberculosis without any clinical symptoms, radiological abnormality, or microbiological evidence1. One-third of the world's population is infected with TB, and the prevalence rate of LTBI in low- or middle-income countries is estimated to be as high as 51.5%, while that in high-income countries is 28.1%2.
Active TB has a greater burden of TB bacilli than latent TB, and acts as an infection source for contacts. Preventing TB infection by bacillus Calmette–Guérin vaccination, and detecting and treating early-stage TB in high-burden countries through an active case-finding strategy are used as the main strategies for eradicating TB. Conversely, an active treatment strategy for LTBI is used in low TB-burden countries. Human immunodeficiency virus–infected patients or household TB contacts <6 years of age are candidates for LTBI treatment in high TB-burden countries2. However, the main candidates for LTBI treatment in low TB-burden countries are immigrants from TB-endemic areas23. Moreover, it has been reported that most new TB diagnoses originate from progression of recent LTBI rather than remote TB reactivation45. Diagnosis and treatment for LTBI is a major strategy for reducing TB prevalence, but it must be pursued on the precondition that active TB is not missed. Investigation of close household contacts is supposed to reduce TB prevalence by 2% per year2. This review will cover the principles of TB infection and transmission from basic and clinical perspectives as well as the broad outlines for TB infection control to reduce TB transmission.
TB bacilli can be transmitted as droplet nuclei that are residues of dried respiratory droplets. Droplet nucleus that contain 1–10 TB bacilli is 1–5 µm in diameter. These droplets can remain in the air for several hours and can be inhaled into the alveoli. On the other hand, respiratory droplets that are >100 µm in diameter will fall to the ground within 1 m of the origin, and will be impacted to the upper airway. Typical droplet nuclei-derived infections are measles and TB, while respiratory droplets cause staphylococcus and respiratory syncytial virus infections. Fluid lining the respiratory tract and TB bacilli contained in it may become aerosolized by high-velocity airflow during coughing or sneezing. Moreover, implantation of TB bacilli from droplet nuclei can occur after aerial transportation, even though the infectiousness of TB is relatively weaker than that of measles. Respiratory secretions surrounding droplet nuclei variably protect TB bacilli from dehydration, oxygen injury, natural irradiation, and other environmental stresses. Experiments have revealed that the half-life of aerosolized TB bacilli is about 6 hours6.
Laboratory experiments using guinea pigs as quantitative air samplers for human TB in TB wards with six single rooms proved the following five important facts about TB transmission7: (1) TB is a true airborne infection, requiring only air contact for transmission, (2) TB patients vary greatly in infectiousness, (3) infectiousness is rapidly reduced by effective treatment, (4) the average concentration of infectious droplet nuclei is low, and (5) ultraviolet irradiation is a highly effective method of air disinfection. TB bacilli are engulfed by alveolar macrophages, and infection appears to be aborted without immunological stigma if the organisms are destroyed before they replicate to 10–15 generations8. Transmission of TB bacilli is influenced by strength of the infectious source, infectiousness of virulent TB bacilli, immunoresistance of the host, environmental stresses, and biosocial factors, as shown in Figure 18.
The most representative quantitative study for airborne infection through droplet nuclei is the school measles outbreak study. However, Edward Riley modified Wells's use of the Soper mass balance equation9 for TB ward experiments10 as follows, while TB infectiveness through casual contact was assumed to be relatively lower than that of measles: , where, C=Number of new cases, S=Number of susceptibles exposed, e=Natural logarithm, I=Number of infectious sources, q=Number of quanta (infectious doses) generated per unit min, p=Human ventilation rate (L/min), t=Exposure duration, Q=Infection-free ventilation (L/sec) (assumption: uniform susceptibility of exposed persons to infection, uniform virulence of organisms from one outbreak to another).
According to experiments using guinea pigs and inbred rabbits, it was reported that a single droplet nucleus containing more than three TB bacilli is enough to cause TB infection11. Although TB susceptibility varies among different human races and individuals, it is estimated to be nearly the same as in the animal experiments.
Recently, TB outbreaks have been reported frequently in middle and high schools, military locations, work places, and dormitory settings in South Korea12. Therefore, LTBI treatment has become an important policy for reducing the TB prevalence rate in South Korea, and LTBI control guidelines have been strengthened by the revision of the 2014 Korean guidelines for TB13. Many guidelines for TB infection control have been published internationally as follows: Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings (US Centers for Disease Control and Prevention, CDC)14; World Health Organization (WHO) Policy on TB Infection Control in Health-Care Facilities, Congregate Settings, and Households (WHO)15; Guidelines for Design and Construction of Health-Care Facilities (American Institute of Architects)16; and additional updates for personal protective equipment guidelines (US Occupational Safety and Health Administration, 2003). WHO guidelines for TB infection control describe national and subnational activities as managerial activities, administrative control, engineering control, environmental control, and personal protective equipment provision for the implementation of TB infection control in health-care facilities, congregate settings, and households, respectively (Table 1)15.
Guidelines regarding managerial activities provide policy makers at the national and subnational levels with a comprehensive framework that can support and facilitate the implementation, operation, and maintenance of TB infection control in health-care facilities, congregate settings, and households. Administrative controls mean activities that are intended to reduce the risk of exposure to persons with infectious TB. These controls include policies and procedures for the early identification, evaluation, isolation, and treatment of patients likely to have TB. Environmental controls mean the use of engineering technologies to reduce the concentration of airborne infectious droplet nuclei to prevent the spread of TB. Personal protective equipment is the last level of TB control and concerns the use of respiratory protection that can reduce the risk of exposure to infectious droplet nuclei for health-care workers. The U.S. Centers for Disease Control and Prevention propose guidelines for inpatient settings (patient rooms, emergency departments, intensive care units, surgical suites, laboratories, bronchoscopy suites, sputum induction or inhalation therapy rooms, autopsy suites, and embalming rooms), outpatient settings (TB treatment facilities, medical offices, ambulatory-care settings, dialysis units, and dental-care settings), and nontraditional facility-based settings (medical settings in correctional facilities, long-term care settings, and emergency medical services)14. Homeless shelters should follow the same TB infection control measures, but LTBI screening for homeless people is not recommended because of concerns about cost-effectiveness and compliance17. New immigrants from countries with a high TB incidence must also be screened for active TB18.
Patients with pulmonary, laryngeal, or endobronchial TB are highly contagious, and the infectiousness is increased in the following conditions: (1) presence of lung cavities, (2) positive acid-fast bacillus smear or culture for sputum, and (3) presence of respiratory symptoms like cough. On the other hand, infectiousness is rapidly reduced by anti-TB chemotherapy. TB bacilli burden is reduced to 1/25th of the initial level within 2 days of anti-TB chemotherapy, and to 1/100th within 2–3 weeks of anti-TB chemotherapy in drug-sensitive TB patients1920. The infectious period is over when the following criteria are satisfied: (1) effective treatment (as demonstrated by M. tuberculosis susceptibility results) for >2 weeks, (2) diminished symptoms, and (3) mycobacteriologic response (e.g., decrease in grade of sputum smear positivity detected on sputum-smear microscopy). Even though the start of the infectious period cannot be determined with precision, an assigned start that is 3 months before a TB diagnosis is recommended based on the characteristics of an index TB patient (Table 2)20.
The risk of TB infection of contacts is different depending on the proximity and duration of contact. TB contacts are classified as close and casual contacts according to the intensity of contact. Close contacts are those who spend a long time (more than 8 hours) with infectious TB patients in a closed space18; they are further divided into close household and close non-household contacts. Close household contacts are those who share the patient's breathing space daily (bedroom, kitchen, bathroom, or living room) and live with infectious TB patients. Close non-household contacts are those who share breathing space with an index TB patient outside the household (e.g., workplace, school, etc.). In contact investigation, higher priority is given to close contacts including household contacts as well as to risk groups; casual contacts are the next priority. When M. tuberculosis is inhaled, alveolar macrophages kill the TB bacilli. When humans are infected with M. tuberculosis , 10% of infected hosts progress to active TB and the remaining 90% sustain LTBI. High-risk groups such as human immunodeficiency virus–infected patients and contacts <2 years old, and moderate-risk groups such as children and adolescents between 2–18 years of age and patients with end-stage renal disease or diabetes mellitus can easily progress to active TB if exposed to infectious TB. Therefore, these groups must have higher priority for the diagnosis of LTBI (Figure 2)21.
Cell-mediated immune response develops 2–10 weeks after infection by M. tuberculosis and is identified by tuberculin skin test conversion; this accelerates granuloma formation in the body22. The infected person can show no clinical manifestation or mild respiratory symptoms including febrile sensations. In primary TB disease (recent infection), some young children can show infiltration on chest radiographs suggesting active TB, but this manifestation is frequently spontaneously resolved leaving calcified scar lesions. In contrast, reactivation of latent TB caused by a weakened immune status and presenting as cavities in the lung apex or superior segment of the lower lobe is known as secondary TB disease (remote infection). However, according to recent reports, different presentations of primary TB disease, which shows pulmonary infiltration at the lower lobe, and secondary TB disease, which presents as pulmonary cavities in the apex or superior segment, seem to be related to the host's immune status rather than time23. It seems that active TB generally develops within a year after LTBI, and the rate of active TB cases decreases sharply after the first year and continues to decrease slowly thereafter in the following 10 years24. Meanwhile, pleural TB, meningeal TB, and military TB seem to be reactivated in the earlier period. In addition, it was reported that previous TB exposure provides protective immunity against further infection (reinfection) in 16%–40% of cases25.
There is a subclinical borderline phase with uncertain radiological manifestations prior to symptomatic presentation26. In this obscure subclinical phase, which can last for months between latent and active clinical TB, molecular diagnostic methods or computerized tomography scans can be used to prevent infectious TB from going unnoticed27, but cost-effectiveness must also be considered. To reduce the TB prevalence rate effectively, the range of candidates for LTBI treat-ment must be expanded. However, treatment interruption and overtreatment for unnecessary candidates must be avoided. In other words, shorter treatments with fewer adverse events must be performed and the number of candidates needed to be treated for preventing one additional active TB progression from latent TB must be minimized by mutual agreement between policymakers and clinicians26.
TB can be transmitted by respiratory droplet nuclei. The factors that influence the transmission of TB bacilli are the strength of the infectious source, infectiousness of virulent TB bacilli, immunoresistance of the host, environmental stresses, and biosocial factors. Infection controls to reduce TB transmission consist of national and subnational activities, which include managerial activities, administrative control, engineering control, environmental control, and personal protective equipment provision, in health-care facilities, congregate settings, and households, respectively. Active TB progression from LTBI is influenced by the age and immune status of the host. To reduce the TB prevalence rate, the range of candidates for LTBI treatment, including those in the high-risk group, needs to be expanded. However, consensus between clinical and public aspects must be achieved based on definite domestic evidences.
Figures and Tables
Figure 1
Schematic presentation of factors determining the likelihood of transmitting tuberculosis (TB) infection8. BCG: bacillus Calmette–Guérin.
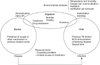
Figure 2
Schematic diagram for latent tuberculosis infection (LTBI) screening in tuberculosis (TB) contacts and noncontacts in Korea21.

Table 1
Activities to reduce the transmission of TB15

Table 2
Guidelines for estimating the beginning of the infectious period of TB patients according to index characteristics20

References
1. Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003; 362:887–899.
2. Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet. 2016; 387:1211–1226.
3. Lillebaek T, Andersen AB, Dirksen A, Smith E, Skovgaard LT, Kok-Jensen A. Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerg Infect Dis. 2002; 8:679–684.
4. Alland D, Kalkut GE, Moss AR, McAdam RA, Hahn JA, Bosworth W, et al. Transmission of tuberculosis in New York City: an analysis by DNA fingerprinting and conventional epidemiologic methods. N Engl J Med. 1994; 330:1710–1716.
5. Small PM, Hopewell PC, Singh SP, Paz A, Parsonnet J, Ruston DC, et al. The epidemiology of tuberculosis in San Francisco: a population-based study using conventional and molecular methods. N Engl J Med. 1994; 330:1703–1709.
6. Loudon RG, Bumgarner LR, Lacy J, Coffman GK. Aerial transmission of mycobacteria. Am Rev Respir Dis. 1969; 100:165–171.
7. Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, et al. Aerial dissemination of pulmonary tuberculosis: a two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995; 142:3–14.
8. Reichman LB, Hershfield ES. Tuberculosis: a comprehensive international approach. 2nd ed. New York: Marcel Dekker Inc.;2005.
9. Riley EC, Murphy G, Riley RL. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 1978; 107:421–432.
10. Riley RL. Aerial dissemination of pulmonary tuberculosis. Am Rev Tuberc. 1957; 76:931–941.
11. Ratcliffe HL, Palladino VS. Tuberculosis induced by droplet nuclei infection; initial homogeneous response of small mammals (rats, mice, guinea pigs, and hamsters) to human and to bovine bacilli, and the rate and pattern of tubercle development. J Exp Med. 1953; 97:61–68.
12. Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015; 21:1913–1920.
13. Lee SH. Diagnosis and treatment of latent tuberculosis infection. Tuberc Respir Dis. 2015; 78:56–63.
14. Jensen PA, Lambert LA, Iademarco MF, Ridzon R. CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005; 54:1–141.
15. World Health Organization. WHO policy on TB infection control in health-care facilities, congregate settings and households. Geneva: World Health Organization;2009.
16. Ninomura P, Rousseau C, Bartley J. Updated guidelines for design and construction of hospital and health care facilities. ASHRAE J. 2006; 48:H33–H37.
17. Canadian Thoracic Society. Canadian tuberculosis standards. 7th ed. Ottawa: Canadian Thoracic Society;2013.
18. National Institute for Health and Clinical Excellence. Clinical guideline 33. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control [Internet]. London: National Institute for Health and Clinical Excellence;2011. cited 2016 Apr 2. Available from: http://www.nice.org.uk/CG117.
19. Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003; 167:1348–1354.
20. National Tuberculosis Controllers Association. Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005; 54:1–47.
21. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2014.
22. Allen EA. Tuberculosis and other mycobacterial infections of the lung. In : Thurbeck WM, Chung AM, editors. Pathology of the lung. 2nd ed. New York: Thieme Medical Publishers;1995. p. 253–254.
23. Elkington PT, Friedland JS. Permutations of time and place in tuberculosis. Lancet Infect Dis. 2015; 15:1357–1360.
24. Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Bibl Tuberc. 1970; 26:28–106.
25. Brooks-Pollock E, Becerra MC, Goldstein E, Cohen T, Murray MB. Epidemiologic inference from the distribution of tuberculosis cases in households in Lima, Peru. J Infect Dis. 2011; 203:1582–1589.
26. Esmail H, Barry CE 3rd, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130437.
27. Ryu YJ. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis. 2015; 78:64–71.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download