Abstract
Tuberculosis (TB) is one of the most important occupational risks for healthcare workers (HCWs) in South Korea. Many policies regarding the control and prevention of TB in healthcare settings recommend that HCWs are tested for latent tuberculosis infection (LTBI) in addition to active TB. Moreover, the Korean Tuberculosis Prevention Act also recommends that HCWs receive regular testing for LTBI. However, there are no specific or detailed guidelines for dealing with LTBI in HCWs. Herein, we discuss the diagnosis and treatment of LTBI in HCWs and focus particularly on the baseline screening of hired HCWs, routine follow-up, and contact investigation.
Healthcare workers (HCWs) are more likely to be exposed to people with tuberculosis (TB), so they are at increased risk for developing the disease1. TB is one of the most common occupational infectious diseases among HCWs in South Korea2. A previous study reported that the prevalence of TB for nurses working in TB-related departments was 5.1 times higher than the general population3. In contrast, the prevalence of latent tuberculosis infection (LTBI) is 17%–37% for existing HCWs and 10%–26% for newly hired HCWs in South Korea456. The annual risk of acquiring a TB infection (the conversion rate) among newly hired HCWs is at least 3%, more than 10 times higher than that of the general population67.
To prevent the development of TB among HCWs, a number of national policies and guidelines recommend that HCWs should be included in LTBI screening programs. The Korean Tuberculosis Prevention Act states that all HCWs who attend to TB patients should receive periodic screening for LTBI in addition to active TB. Moreover, the Korean Guidelines for Tuberculosis recommend the regular testing of HCWs for LTBI8. However, to date no guidelines have been developed with specific or detailed recommendations concerning LTBI in HCWs in South Korea.
In this article, we discuss the diagnosis and treatment of LTBI in HCWs. Additionally, we suggest a simplified protocol for LTBI screening for use in various types of healthcare settings.
Baseline testing for LTBI among newly hired HCWs is important because the results provide a basis for comparison in the event of potential or known exposure to Mycobacterium tuberculosis9. The U.S. Centers for Disease Control and Prevention (CDC) recommends baseline testing for M. tuberculosis infection for all newly hired HCWs, regardless of the infection risk classification of the healthcare setting9. Additionally, the U.K. National Health Service (NHS) recommends that all new employees who will be working with patients or clinical specimens should not begin work until they have completed a TB screen or health check10.
In a high or intermediate TB-burden country such as South Korea, many HCWs are tested for LTBI as part of contact investigations or periodic follow-up and, therefore, baseline test results are essential for comparison. All newly hired HCWs should, therefore, receive baseline screening for LTBI before starting work.
There are two diagnostic methods used for LTBI: the Mantoux tuberculin skin test (TST) and the interferon-gamma release assay (IGRA). TST provides a rich source of data and evidence and is inexpensive to perform. However, it has several limitations including providing false-positive results in bacillus Calmette–Guérin (BCG)–vaccinated people, the need for an individual to measure the site of the skin test (i.e., a subjective measure), the requirement for multiple visits, and the bolstering of the immune system response (booster effect). The advantages of IGRA are its specificity, the need for only a single visit, and the lack of a booster effect. However, errors in collecting or transporting blood specimens or in running and interpreting the assay can decrease the accuracy of IGRA, due to the complex laboratory steps required, and it is expensive to perform.
Previous studies have revealed inconsistencies between TST and IGRA results among South Korean HCWs456. These studies all reported a higher positive rate for TST than IGRA, suggesting that TST might provide false-positive results because almost all HCWs in the same studies had received BCG vaccination. Other studies have found that exposure to TB was associated only with positive IGRA results, rather than the results from TST; IGRA would therefore be a more appropriate test for determining LTBI in HCWs4511. In conclusion, IGRA may be better than TST for the baseline screening of LTBI in HCWs in South Korea, a country where HCWs commonly receive the BCG vaccination. However, healthcare providers should consider that IGRA has an important limitation during serial testing (described in detail in "Routine follow-up for LTBI in HCWs" section).
The Korean guidelines for TB recommend that either IGRA or TST can be used to test for LTBI (however, they provide no specific recommendations for HCWs)8. A dual screening strategy (i.e., TST first, followed by IGRA) can also be used. If TST is performed for baseline screening, a two-step TST test (retest 1–4 weeks later in cases where the initial TST result is negative) is required to counter the booster effect. In a previous study, the booster effect was observed in 14.2% of South Korean HCWs12. A second TST is not required if the HCW has a documented TST result from any time during the previous 12 months. A positive TST result is defined as an induration of ≥10 mm in the transverse diameter on either the initial or second TST. If a dual screening strategy for baseline screening is performed, it is preferable to perform IGRA on the day the subject returns for his/her TST reading to reduce the effects of TST on the IGRA test. Baseline screening for LTBI is not necessary for HCWs who have written documentation of either a previous positive TST/IGRA result or treatment for LTBI/TB.
HCWs frequently come into contact with patients and other HCWs in crowded settings. If HCWs develop TB while at work, they are likely to spread M. tuberculosis to a large number of patients and other HCWs, so immunocompromised patients and co-workers may face the risk of developing TB.
A number of guidelines recommend that HCWs should be possible candidates for LTBI treatment upon hire910. However, no detailed recommendations concerning LTBI treatment for newly hired HCWs are available in South Korea. Therefore, it may be appropriate to recommend LTBI treatment to newly hired HCWs at high risk of developing TB according to the Korean Guidelines for Tuberculosis, as in the general population, (details are listed in the table on p. 184 of the Korean Guidelines for Tuberculosis)8. Additionally, HCWs who serve immunocompromised people (e.g., in an organ transplantation unit or newborn nursery) may be considered a candidate for treatment if their baseline result is positive.
Each healthcare provider can decide the appropriate regimen for LTBI treatment from among the following choices, taken from the Korean guidelines for TB8: 9 months of isoniazid treatment (9H), 4 months of rifampin (4R), or 3 months of both isoniazid and rifampin (3HR).
According to Article 11 of the Korean Tuberculosis Prevention Act (revised 28 January 2014), all HCWs who serve patients with TB should be periodically tested for LTBI, and this should occur at least once a year. However, it does not provide specific guidelines for identifying candidates for screening or frequency of screening.
The CDC states that the screening frequency should be based on the risk classification of various healthcare settings, and that these are determined by the type of hospital/treatment facility (e.g., in/outpatient setting, number of beds, whether it is a TB treatment facility or laboratory) and the number of TB patients treated over the preceding year9. These guidelines recommend that routine follow-up is not necessary for low-risk hospital settings; that HCWs should receive annual screening for LTBI in settings classified as medium risk; and that if the setting is classified as one where there is a potential for ongoing transmission (i.e., evidence of person-to-person transmission of M. tuberculosis: e.g., unrecognized TB in patients or HCWs), testing for LTBI might need to be performed as frequently as every 8–10 weeks.
However, the CDC recommendations were prepared for a low-incidence country. TB incidence and the healthcare setting in South Korea are much different from those in the United States. Actually, if the CDC recommendations were applied in South Korea, many hospitals would fall into the "potential ongoing transmission" category; as such, it would be impossible to test all HCWs every 8–10 weeks. It would be more appropriate, instead, to determine the frequency of, and candidates for, screening based on the risk of each department. For example, high-risk departments can be identified based on previous research and recognized guidelines2391314. Table 1 presents risk classification according to department along with the accompanying screening frequency. HCWs in medium-risk departments may be considered for participation in routine follow-up LTBI screening if resources are sufficient. No HCWs work in low-risk departments (setting in which exposure to M. tuberculosis is unlikely) based on the current South Korean situation. HCWs who serve immunocompromised patients may be included in the high-risk category. It should be noted that the classification of high-risk departments can be modified according to the situation and resources of the institution.
Additional LTBI testing does not need to be performed for HCWs who have a documented previous positive test result for M. tuberculosis infection, or documented completion of treatment for LTBI or TB. These employees need only receive a symptom screen and chest radiograph.
Either TST or IGRA can be used for follow-up testing, whichever was previously used for the baseline test. However, IGRA has a number of limitations when used as a serial follow-up test. IGRA is a highly dynamic test and its results frequently fluctuate. It also has poor repeatability and reproducibility. The most important limitation in terms of serial testing is that there is no current acceptable definition for the terms 'conversion' and 'reversion.' As a result, false positive conversion is common in IGRA.
In a previous study conducted in the United States, 6.1% and 8.3% of the total number of HCWs who underwent testing showed conversion with QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB (T-SPOT) testing, respectively, in a serial follow-up test during the study period15. However, of those QFT-GIT and T-SPOT converters, 76.4% and 77.1%, respectively, were found to be negative when retested 6 months later. In contrast, the TST conversion rate in a serial follow-up test was only 0.9%. Additionally, TB was not found to have developed among any of the HCWs during the study period. The authors concluded that most IGRA conversions among HCWs in settings with a low incidence of TB were likely false positives. A separate study of South Korean HCWs obtained similar results16. When HCWs in contact with TB patients were tested monthly by QFT-GIT, results were inconsistent for 52% of the subjects in a serial test. When conversion was defined as a change from a negative, <0.35 IU/mL, to a positive, ≥0.70 IU/mL, results were inconsistent in 27.0% of the subjects. Additionally, some previous studies have reported that IGRA conversion rates are not affected by the department, job category, or risk group according to TB exposure6.
In conclusion, the use of IGRA for the serial testing of HCWs is complicated by a lack of data on the optimum cut-offs for serial testing and an unclear interpretation and prognosis of conversions and reversions. Therefore, caution is urged when using IGRA as a serial follow-up test. When using TST for follow-up testing, TST conversion is defined as a baseline TST <10 mm and a follow-up TST ≥10 mm, with an increment ≥6 mm.
All HCWs with newly positive results on routine follow-up testing (converters) should be considered for treatment for LTBI, regardless of age. If HCWs with positive LTBI test results on routine follow-up do not have previous or baseline test results, it may be appropriate to consider treatment if they are at high risk for developing TB8. Any of the treatment regimens described above are acceptable, i.e., 9H, 4R, or 3HR8.
In this section, we focus on the scenario where HCWs are exposed to patients with TB and HCWs are diagnosed or suspected of having TB.
An index patient must be defined to initiate a contact investigation. The CDC defines an index patient as one who provides a positive sputum acid-fast bacilli (AFB) smear, a M. tuberculosis culture, or a positive TB polymerase chain reaction (PCR) result. It also recommends that a patient who only receives a positive result from bronchoalveolar lavage (BAL) fluid or gastric aspirate and who reveals an abnormal chest radiograph consistent with TB but without evidence of bacteria is also classed as infectious17. In contrast, some U.K. guidelines regard a patient as infectious if only a sputum AFB smear result is returned as positive (except for multi-drug-resistant TB, aerosol-producing procedures, and immunocompromised hosts). They recommend that a positive result from bronchial washing is treated as non-infectious18. The Korean guidelines for TB control (although these guidelines are not specific for HCWs) define an index patient as one who provides a positive sputum AFB smear, M. tuberculosis culture, or positive TB PCR result, or a compatible chest radiograph suggestive of TB. A positive result from washing or BAL fluid is also regarded as indicating infection19.
It is difficult to determine the precise start of the infectious period with currently available methods, so it is necessary to provide an estimate17. The CDC recommends that for patients exhibiting symptoms of TB, providing a positive sputum AFB stain result, or showing cavities on a chest radiograph, the earliest starting point of the infectious period is either 3 months prior to the onset of symptoms or the date of the first positive finding consistent with TB disease; if patients have none of these, the start of the period should be considered to be 4 weeks prior to the date of the suspected diagnosis17. The Korean guidelines for TB control recommend the same infectious period as that of the CDC19.
For optimal effectiveness, a contact investigation should be performed as a priority. When evidence of a new M. tuberculosis infection among high-priority contacts is discovered, the contact investigation should then be extended to the mediump-riority contacts. High-priority contacts include close contacts, contacts who are exposed during medical procedures (e.g., bronchoscopy, sputum induction, and otorhinolaryngeal examination), and non-close contacts with a medical risk factor for developing TB, regardless of age.
Unfortunately, the guidelines do not clearly define what a 'close contact' is. The CDC suggests that a close contact is a person who has shared the same air space in a household or other enclosed environment for a prolonged duration (days or weeks, rather than minutes or a few hours) with someone suspected of, or confirmed with, TB9. The NHS states that there is no clear definition of a close contact and it is therefore difficult to give guidance about who to trace. However, it also notes that people should be regarded as at risk of infection if they have spent more than 8 hours in the same hospital bay with an inpatient with sputum smear-positive TB who had a cough10. On the other hand, the Tuberculosis Network European Trials Group recommends that close nonhousehold contacts are those who are exposed for a cumulative time of 8 hours, if the index is sputum smear-positive, or 40 hours, if only sputum culture-positive20.
Because a clear definition of a 'close contact' does not yet exist, HCWs who are part of a contact investigation should be selected on a case-by-case basis according to the risk of infection, as follows: (1) intensity of exposure based on proximity, (2) overlap with the infectious period of the index case, (3) duration of exposure, (4) presence or absence of infection-control measures, (5) infectiousness of the index case, and (6) performance of procedures that could increase the risk for transmission during contact (e.g., sputum induction, bronchoscopy, and airway suction)9.
HCWs with a previous negative or unknown LTBI test result should undergo either TST or IGRA testing (whichever was used for the previous test) as well as a symptom screen and chest radiograph. HCWs with a documented previous positive LTBI test result, a documented history of TB, or documented completion of treatment for LTBI or TB should receive a symptom screen and chest radiograph without the need for a further LTBI test.
All contacts with newly positive results (converters) should be considered for treatment for LTBI, regardless of age. However, the exclusion of active TB is essential before the commencement of LTBI treatment. Contacts with documented previous positive LTBI test results, a documented history of TB, or documented completion of treatment for LTBI or TB may be considered for treatment depending on the medical risk factors and intensity of exposure. If HCWs with a positive LTBI test result from the contact investigation do not have previous or baseline test results, it may be appropriate to apply the same indications as for the general population according to the Korean Guidelines for Tuberculosis (details listed in table on p. 186 of the Korean Guidelines for Tuberculosis)8. As previously, any of the treatment regimens discussed above are acceptable, i.e., 9H, 4R, or 3HR8.
The Korean guidelines for TB control recommend that a contact investigation should be initiated immediately if HCWs are diagnosed or suspected of having TB19. High-priority contacts include co-workers who have shared the same air space for >8 hours. Patients who have been served by HCWs are also high priority. All contacts should receive symptom screening and a chest radiograph. Also, contacts with previous negative or unknown LTBI test results should undergo either the TST or IGRA testing. In congregated settings, such as hospitals, a repeat TST or IGRA 8–10 weeks after exposure is recommended if the initial TST or IGRA result is negative19. The overall LTBI treatment processes are similar to those described above for HCW contacts. Contacts who are patients should be considered to treat for LTBI according to the Korean guidelines for TB and the Korean guidelines for TB control819.
HCWs with confirmed infectious TB disease should be excluded from the workplace and should return to work only after the following criteria have been met (1) three consecutive negative sputum samples collected at 8–24-hour intervals, with at least one early morning sample; (2) responded to anti-TB treatment that will probably be effective; and (3) determined to be noninfectious by a physician knowledgeable and experienced in managing TB disease9.
All newly hired HCWs should receive baseline testing for LTBI before commencing employment, regardless of their work risk category. Additionally, HCWs who work in high-risk departments should receive regular follow-up screening for LTBI in addition to that already in place for active TB. When HCWs come into contact with a TB patient, a contact investigation should be undertaken immediately. Figure 1 presents a flowchart of baseline and follow-up testing for LTBI. To enable the effective control and prevention of TB in healthcare settings in South Korea, specific and detailed guidelines for dealing with LTBI in HCWs are required.
Figures and Tables
Figure 1
Flowchart of baseline and follow-up testing for latent tuberculosis infections in healthcare workers. *Routine follow-up latent tuberculosis infection screening for medium risk departments may be considered if resources are sufficient. †Use of the IGRA for serial testing is complicated by a lack of optimum cut-off data for serial testing. Caution is urged when using the IGRA as a follow-up test. HCWs: healthcare workers; Sx: symptom; CXR: chest radiograph; TST: Mantoux tuberculin skin test; IGRA: interferon-gamma release assay; TB: tuberculosis; LTBI: latent tuberculosis infection; Hx: history.
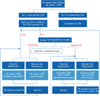
Table 1
Risk classification and latent tuberculosis infection screening frequency
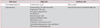
HCWs who serve immunocompromised persons (e.g., in an organ transplantation unit or newborn nursery) may be considered to be high risk.
*HCWs who are in medium-risk departments can be considered for participation in routine follow-up latent tuberculosis infection screening if resources are sufficient.
TB: tuberculosis; ICU: intensive care unit; PPM: public-private mix; HCWs: healthcare workers.
References
1. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000; 49:1–51.
2. Ahn YS, Lim HS. Occupational infectious diseases among Korean health care workers compensated with Industrial Accident Compensation Insurance from 1998 to 2004. Ind Health. 2008; 46:448–454.
3. Jo KW, Woo JH, Hong Y, Choi CM, Oh YM, Lee SD, et al. Incidence of tuberculosis among health care workers at a private university hospital in South Korea. Int J Tuberc Lung Dis. 2008; 12:436–440.
4. Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis. 2013; 75:18–24.
5. Lee KJ, Kang YA, Kim YM, Cho SN, Moon JW, Park MS, et al. Screening for latent tuberculosis infection in South Korean healthcare workers using a tuberculin skin test and whole blood interferon-gamma assay. Scand J Infect Dis. 2010; 42:672–678.
6. Park HY, Jeon K, Suh GY, Kwon OJ, Chung DR, Yoonchang SW, et al. Interferon-gamma release assay for tuberculosis screening of healthcare workers at a Korean tertiary hospital. Scand J Infect Dis. 2010; 42:943–945.
7. Lee K, Han MK, Choi HR, Choi CM, Oh YM, Lee SD, et al. Annual incidence of latent tuberculosis infection among newly employed nurses at a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009; 30:1218–1222.
8. Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention;2014.
9. Jensen PA, Lambert LA, Iademarco MF, Ridzon R. CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005; 54:1–141.
10. National Institute for Health and Care Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control [Internet]. London: National Institute for Health and Care Excellence;2011. cited 2011 Mar 23. Available from: https://www.nice.org.uk/guidance/cg117/chapter/1-Guidance.
11. Kim SY, Park MS, Kim YS, Kim SK, Chang J, Kang YA. Conversion rates of an interferon-gamma release assay and the tuberculin skin test in the serial monitoring of healthcare workers. Infection. 2013; 41:511–516.
12. Kim SY, Park MS, Kim YS, Kim SK, Chang J, Yong D, et al. Tuberculin skin test and boosted reactions among newly employed healthcare workers: an observational study. PLoS One. 2013; 8:e64563.
13. Kim SJ, Lee SH, Kim IS, Kim HJ, Kim SK, Rieder HL. Risk of occupational tuberculosis in National Tuberculosis Programme laboratories in Korea. Int J Tuberc Lung Dis. 2007; 11:138–142.
14. Operational Directives and Information Circulars. Tuberculosis and health care workers [Internet]. East Perth: Operational Directives and Information Circulars;2011. cited 2011 Sep 2. Available from: http://www.health.wa.gov.au/circularsnew.
15. Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, et al. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014; 189:77–87.
16. Park JS, Lee JS, Kim MY, Lee CH, Yoon HI, Lee SM, et al. Monthly follow-ups of interferon-gamma release assays among health-care workers in contact with patients with TB. Chest. 2012; 142:1461–1468.
17. National Tuberculosis Controllers Association. Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. 2005; 54:1–47.
18. Frances S, Katrina W. Guidelines for the prevention and control of tuberculosis in hospital [Internet]. Watford: West Hertfordshire Hospitals;2013. cited 2013 Feb 1. Available from: http://westhertshospitals.nhs.uk/infection_control/documents/policies/TB_Policy_Feb_2013_v7.pdf.
19. Ministry of Health and Welfare and Korean Centers for Disease Control and Prevention. National guidelines for tuberculosis control, 2015. Seoul and Cheongwon: Ministry of Health and Welfare, Korean Centers for Disease Control and Prevention;2015.
20. Erkens CG, Kamphorst M, Abubakar I, Bothamley GH, Chemtob D, Haas W, et al. Tuberculosis contact investigation in low prevalence countries: a European consensus. Eur Respir J. 2010; 36:925–949.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download