Abstract
Orthostatic hypotension (OH) occurs when mechanisms for the regulation of orthostatic BP control fails. Such regulation depends on the baroreflexes, normal blood volume, and defenses against excessive venous pooling. OH is common in the elderly and is associated with an increase in mortality rate. There are many causes of OH. Aging coupled with diseases such as diabetes and Parkinson's disease results in a prevalence of 10-30% in the elderly. These conditions cause baroreflex failure with resulting combination of OH, supine hypertension, and loss of diurnal variation of BP. The treatment of OH is imperfect since it is impossible to normalize standing BP without generating excessive supine hypertension. The practical goal is to improve standing BP so as to minimize symptoms and to improve standing time in order to be able to undertake orthostatic activities of daily living, without excessive supine hypertension. It is possible to achieve these goals with a combination of fludrocortisone, a pressor agent (midodrine or droxidopa), supplemented with procedures to improve orthostatic defenses during periods of increased orthostatic stress. Such procedures include water bolus treatment and physical countermaneuvers. We provide a pragmatic guide on patient education and the patient-orientated approach to the moment to moment management of OH.
Orthostatic intolerance refers to the development of symptoms such as lightheadedness and blurred vision when a subject stands up that clears on sitting back down. Other symptoms include cognitive blunting, tiredness, and head and neck ache. These symptoms are due to cerebral hypoperfusion.1 The posterior head and neck ache (with a coathanger distribution) is thought to be due to ischemia of large neck muscles.1 Other symptoms such as palpitations, tremulousness, nausea, and vasomotor changes are due to sympathetic hyperactivity and occur in patients with only partial autonomic failure.
Not every patient with orthostatic intolerance has orthostatic hypotension (OH). Common causes of orthostatic intolerance are shown in Table 1. A common cause of transient orthostatic intolerance is reflex syncope (vasovagal, vasodepressor). An otherwise normal person suddenly faints. Vasovagal and vasodepressor syncope are both characterized by the sudden abrupt fall in blood pressure (BP). They differ in that in vasovagal syncope, the abrupt fall in BP, is accompanied by a similarly abrupt fall in heart rate whereas the latter is absent in vasodepressor syncope. They are often triggered by pain (such as receiving an injection or blood-draw) or emotional stimulus. These occur in persons with normal baroreflexes and occur suddenly. Another cause, occurring about 5-10 times as commonly as OH, is postural tachycardia syndrome, characterized by orthostatic intolerance coupled with orthostatic tachycardia.2 OH is defined as a reduction of systolic BP of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg within 3 minutes of standing up.3
The "prevalence" of OH increases with age and occurs in 10-30% of elderly persons.4 There is a moderate spread in reported frequency of OH (Table 2). Although the values are not population based, and therefore not true prevalences, the numbers are pragmatically important. They make the point that OH in the elderly is common. BP control becomes progressively more impaired with aging, due to a multitude of reasons including impaired baroreflex sensitivity, volume status, and venomotor tone.4 Part of the explanation resides in the increased occurrence of associated conditions like diabetes and Parkinson's disease as well as the effects of drugs like anti-hypertensive agents, diuretics, and anti-Parkinsonian drugs like levodopa. The presence of OH is associated with increased mortality and morbidity.4 The reason for the increase in morbidity and mortality is multifold but includes the consequences of repeated falls, resulting in fractures, head injury, and their complications.
The normal human subject maintains the same BP supine and standing. This maintenance of postural normotension depends on a normal plasma volume, intact baroreflexes, and reasonable venomotor tone.5 A subject with reduced plasma volume (hypovolemia) can develop OH. Similarly, OH can occur in some subjects (predisposed to OH) because of excessive venous pooling. The splanchnic mesenteric bed is especially important because of its large volume and baroreflex responsiveness.67 Splanchnic-mesenteric volume increases 200-300% after a meal7 and this increased venous capacitance causes venous pooling with resultant post-prandial OH in predisposed subjects. Standing in normal subjects results in a fall in blood and pulse pressure and this fall is sensed by baroreceptors in carotid sinus and aortic arch.5 Baroreceptor afferents synapse at the nucleus of the tractus solitaries (Fig. 1). The vagal baroreflex pathway runs from the nucleus of the tractus solitarius to the nucleus ambiguus and sends efferents to the sinoatrial node to increase heart rate. The adrenergic baroreflex pathway runs from the nucleus of the tractus solitarius to the caudal ventrolateral medulla and from there to the rostral ventrolateral medulla. The adrenergic pathway continues with sympathetic efferents from the rostral ventrolateral medulla to the interomediolateral column of the thoracic spinal cord, and from there to autonomic ganglia and to the heart, arterioles, and venules. Hence, the initial fall in BP is corrected by an increase in HR and total systemic resistance. If the baroreflexes fail, as in adrenergic autonomic failure, there are several consequences. There is:
a. OH.
b. Supine hypertension (since baroreflexes also prevent excessive BP increase).
c. Loss of diurnal BP variation. The normal subject has lower nocturnal BP. Patients with baroreflex failure have unchanged or higher nocturnal BP.
There are many causes of OH (Table 3). Most cases seen in clinical practice are best divided into those with and without CNS involvement. Patients with CNS involvement can be separated into those with brain or spinal cord disease. Patients with chronic OH without CNS involvement will most commonly have OH due to diabetes. Less likely causes are amyloid, either sporadic or inherited (tranthyretin mutation), autoimmune, or paraneoplastic etiology. Some have no cause found and are typically designated as idiopathic OH or pure autonomic failure. If they have dream enactment behavior, they are best designated pure autonomic failure, since they likely have a synucleinopathy.
In a setting of acute onset of OH, the main considerations are Guillain-Barre syndrome, where the diagnosis is usually obvious because of severe motor weakness, respiratory compromise, and acute autoimmune autonomic neuropathy ("acute pandysautonomia"). The latter is characterized by severe and generalized autonomic failure without prominent motor or sensory involvement. Other causes such as botulism, porphyria, or those due to toxic causes are uncommon. Their consideration comes up if there is an acute autonomic neuropathy that is undiagnosed and especially if there are red flags for these diseases. In such circumstances, tests such as urine drug and heavy metal screen, tests for porphyria, botulism, and paraneoplastic panel are done.
Chronic causes of OH are much more common than acute causes. The most common cause is mild OH due to old age (discussed earlier). For patients with brain involvement, OH is common in Parkinson's disease, occurring in 20-40 percent of patients, but is usually mild.8 More severe OH occurs in patients with multiple system atrophy or Lewy body dementia.9 Patients with alcoholic neuropathy usually do not have OH and those who have florid OH often have Wernicke-Korsakoff syndrome, with involvement of brain stem autonomic structures.10 Patients with baroreflex failure, as occurs in neck radiation or familial dysautonomia, can have OH, but this symptom is mild compared with autonomic storms, due to de-afferentation.11 Most forms of olivopontocerebellar atrophies do not have OH and patients with chronic cerebellar involvement and OH should raise suspicion of the cerebellar subtype of MSA (MSA-C).
It is desirable to determine if OH is neurogenic, i.e., due to a neurologic basis and not due to hypovolemia or venous pooling. Tests that are helpful in the evaluation of patients are an autonomic reflex screen, thermoregulatory sweat test, 24-hour urinary sodium, and supine and standing plasma norepinephrine. The paraneoplastic panel provides a full battery of antibodies and should be considered if an autoimmune etiology is suspected. Testing to rule out diabetes, amyloidosis (fat aspirate, protein, and immunoelectrophoretogram), porphyria, B12 deficiency, and inherited neuropathy are undertaken as needed.
The autonomic reflex screen is made up of the quantitative sudomotor axon reflex test (QSART), tests of cardiovagal function, and tests of adrenergic function.12 QSART evaluates postganglionic volumes in the forearm and 3 leg sites. We measure heart rate variation and the Valsalva ratio in tests of cardiovagal function.1314 For evaluation of adrenergic reflexes, we evaluate beat-to-beat BP and heart rate responses to the Valsalva maneuver and to head up tilt.15 The autonomic reflex screen will help determine the severity and distribution of sudomotor, cardiovagal, and adrenergic failure. The thermoregulatory sweat test evaluates the distribution of anhidrosis.16 The pattern of anhidrosis can be very helpful. For instance, a length-dependent neuropathy is characterized by distal sweat loss and autoimmune autonomic ganglionopathy by regional loss of sweating whereas pure autonomic failure or MSA might have global anhidrosis. We digitally determine %-anhidrosis, comprising % of anterior body surface that is anhidrotic. Combining thermoregulatory sweet test with QSART can also determine site of the lesion. For instance, if a limb has normal QSART but is completely anhidrotic on TST, the lesion is preganglionic in site.
The 24-hour urinary sodium provides 2 items of useful information. First, it helps determine if the patient is taking the right amount of fluids. The goal is an excretion of 1,500 to 2,500 mL of urine in 24 hours. Second, since sodium is central of maintenance of plasma volume, urine excretion provides verification that the patient is taking enough salt. A urine excretion of >170 mmol/24 hours correlates well with a normal plasma volume.17
Patient education is crucial. The patient is informed on the cause of their OH and management of the cause. For instance, the diabetic needs to optimize blood glucose control. The discussion then moves to the practical management of OH (Table 4). The first 4 items of Table 4 summarize key educational themes that the patient needs to come to terms with. They need to recognize that they have impaired baroreflexes. The consequences of baroreflex failure include OH, supine hypertension, and loss of diurnal variation, since baroreflexes are no longer available to ensure a normal pattern of supine and standing BP.5 Pressor agents such as midodrine are available to raise BP and maintain normal standing BP but at a price of unacceptable supine hypertension.18 The goal is therefore a practical one of providing a moderate pressor effect, sufficient to raise standing BP enough such that the patient has only modest or infrequent symptoms, and has adequate standing time so as to be able to undertake activities of daily living without excessive supine hypertension. Practical values are typically a standing SBP ≥90 mm Hg and supine SBP ≤180 mm Hg. The patient needs to be aware that OH will vary depending on a number of variables, such as volume status; subjects with even transiently low plasma volume, such as on arising, will have lower BP. A meal increases splanchnic mesenteric volume 300%7 and this venous pooling can cause post-prandial OH. Raised ambient heat or a warm bath are potent vasodilators and regularly will aggravate OH. It is important to balance education of the patient on orthostatic stressors with empowering the patient on what they can do to raise BP. They are told that there are 3 variables they can control: plasma volume, venous pooling, and vasomotor tone. They can control these variables with a simple to remember mnemonic.19
Compression of venous capacitance bed reduces venous pooling and orthostatic fall in BP. The largest venous capacitance bed is the splanchnic-mesenteric bed. Hence, compression of this bed by abdominal compression,2021 is much more effective than compressing the leg veins, because of its low volume.21 Jobst stockings are available that compress legs and abdomen, but many patients find the stockings very difficult to apply. A practical alternative is to wear an abdominal binder as a routine. If additional compression is needed, leg stockings are additionally worn.
Water-bolus treatment consists of the patient drinking two 8 ounce glasses of cold water in rapid succession. This results in the abrupt increase in standing systolic blood pressure by about 20 mm Hg for 1-2 hours.2223 The mechanism involves activation of sympathetic adrenergic neurons; plasma concentrations of norepinephrine increase, and the effect can be abolished with trimetaphan, an autonomic ganglionic blocking agent.23 Patients are encouraged to time their water bolus treatment to periods of increased orthostatic stress. For instance, a subject might do one treatment on arising, another before a shopping trip, and a third before exercising. For patients who bolus 3-4 times a day, we advise them to avoid frequent sipping of water, so that they do not get overloaded with water.
Patients with significant supine hypertension are taught to avoid lying flat. They sleep with the head of the bed elevated 4 inches and during the day rest at about a 30 degree angle. They are taught that their normal lying position is 30 degrees from supine. This approach avoids the effects of supine hypertension on brain vessels.
Muscle contraction will raise BP by a muscle pressor response and is the basis of the handgrip test.524 The practical approach is for the patient to contract a group of muscles bilaterally for about 30 seconds, relax, and then repeat the maneuver. Simple maneuvers include standing up on their toes, or crossing their legs and squeezing.19 Some patients manage to unobtrusively contract their buttocks, thighs, and calves while they stand. These maneuvers result in a transient increase in total peripheral resistance.
Midodrine is a directly acting α1-adrenoceptor agonist. It and its active metabolite, desglymidodrine, have a duration of action of 2-4 hours.18 The main side-effects are supine hypertension, paresthesias (including troublesome scalp-tingling), and goose-bumps. Rarely patients develop bladder pain or an inability to void, problems that preclude use of midodrine in those patients.
Fludrocortisone expands plasma volume and increases sensitivity of α-adrenoceptors.25 It is usually used at a dose of 0.1-0.2 mg/day. Main complications are hypokalemia and supine hypertension.25
Droxidopa, an oral norepinephrine precursor, was shown in a phase III treatment trial to improve symptoms and improve standing systolic BP.26 The drug was recently approved by the FDA for rare diseases with OH. Droxidopa is generally well tolerated. It seems to have a duration of action of about 6-8 hours. Currently, midodrine remains the preferred drug. It could potentially be preferable for patients who do not tolerate midodrine or who find its duration of action unacceptably short. Patients with dopamine beta-hydroxylase deficiency seem to have better BP control with droxidopa than midodrine.
Pyridostigmine, a cholinesterase inhibitor, will improve standing BP in patients with OH without aggravating supine hypertension.27 This action occurs since baroreflex unloading occurs on standing and is minimal at rest. Cholinesterase inhibition increases the safety factor of ganglionic transmission by delaying breakdown of acetylcholine. The main limitation of pyridostigmine is its modest effect.
The patient needs to recognize that because they have failure of their baroreflexes, they will no longer have normal BP control. They need to understand what aggravates standing BP and what improves it. For instance OH might be worse first thing in the morning, after a meal, or on a hot day. They need to learn about recognizing subtle symptoms (such as thinking less clearly or feeling tired when they stand). They need to know about techniques to improve OH, such as a bolus or water, countermaneuvers, or venous compression.
Fluid intake of 1.25-2.50 L/day is crucial but is often neglected in elderly people. Salt supplementation is also essential. Most patients manage with salt added to meals but some prefer to use salt tablets (e.g., 0.5 g or 1.0 g tablets). Many patients who have inadequate control of OH have an inadequate salt intake. This can be verified by checking the 24-hour urinary sodium concentration: patients who have a value below 170 mmol can be treated with 1-2 g supplemental sodium three times a day. Urine volume should be between 1,500 and 2,500 mL.
Supine hypertension is common in patients with OH. The best management of supine hypertension is its prevention, by choosing a drug combination coupled with patient education that is sufficient to raise standing BP sufficiently most of the time. The patient can be taught to boost BP transiently during periods of lower BP by approaches such as water bolus and avoidance of autonomic stressors.
Patients are also taught to avoid the supine position. Their new resting position is either the sitting position or lying down at a 30 degree angle during the day and sleep with the head of bed elevated 4 inches at night.
Some patients some of the time will nevertheless still develop florid supine hypertension with sitting BP >180 mm Hg SBP. Management depends on how persistent such a BP is and how responsive it is to simple measures. For instance in some patients, the elevations are transient lasting only half an hour or so and may not require drug treatment. Some patients will enjoy a glass of wine and observe rapid subsidence of hypertension. Some drugs used to treat supine hypertension are shown in Table 5. They are based on the notion that these patients have residual sympathetic tone, which can be blocked with sympathetic antagonists.28 The particular agent selected may depend in part on what action is optimally blocked. For instance in PAF, renin is very low but angiotensin II is paradoxically high and Losartan (an angiotensin II antagonist) will reduce supine hypertension without aggravating early morning OH and decrease nocturnal sodium loss. Losartan 50 mg is given orally about 6 pm. Clonidine is a centrally acting α2 agonist which reduces sympathetic tone. It is usually given at a dose of 0.2 mg and works more gradually, with a delayed onset and longer half-life so that it is often given about meal time. Nitroglycerine patch is simple to use, but some patients are troubled with vascular headaches with the drug. Hydralazine 25 mg or nifedipine 30 mg at night are alternatives. If nocturnal hypotension is an issue, clonidine may be a better option than drugs like nifedipine and hydralazine. Some patients may need a bedside commode to avoid risk of syncope and fall when they walk to the bathroom.
In summary, OH is common, especially in the elderly. Its effects include the risk of falls in the elderly and are associated with an increased mortality rate. Treatment of OH is improving but will not be perfect since baroreflexes are no longer functioning normally. The goal of treatment is to avoid the severe effects of OH and empower the patient to boost defenses against OH during periods of increased orthostatic stress.
Figures and Tables
Fig. 1
Baroreflex pathways for postural normotension. Baroreceptor afferents (dark blue) synapse at the nucleus of the tractus solitarius (NTS). The vagal component of the baroreflex (green) runs from the NTS to the nucleus ambiguus (NA) and sends efferents to the sinoatrial node (SA) to regulate heart rate. The adrenergic baroreflex pathway (red) runs from the NTS to the caudal ventrolateral medulla (CVLM), and from there to the rostral ventrolateral medulla (RVLM). The adrenergic pathway continues with sympathetic efferents from the RVLM to the interomediolateral thoracic spinal cord, and from there to autonomic ganglia and to the heart, arterioles, and venules (Reprinted from Low and Singer.5 Lancet Neurol 2008;7:451-458, with permission from Elsevier).
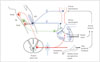
Table 1
Causes of orthostatic intolerance and their differentiation

Table 2
Prevalence of orthostatic hypotension in certain settings

| Setting | Number | Age (years) | Prevalence (%) | Reference |
|---|---|---|---|---|
| Nursing home | 250 | 61-91 | 11 | Rodstein and Zeman29 (1957) |
| Outpatients | 494 | ≥65 | 24 | Caird et al.30 (1973) |
| VA geriatric unit | 319 | 50-99 | 10.7 | Myers et al.31 (1978) |
| Outpatients | 186 | ≥65 | 22 | MacLennan et al.32 (1980) |
| Geriatric unit | 272 | Mean age 83 | 10 | Lennox and Williams33 (1980) |
| Geriatric unit | 247 | ≥60 | 33 | Palmer34 (1983) |
| Outpatients | 300 | Mean age 70 | 6.4 | Mader et al.35 (1987) |
Table 3
Causes of orthostatic hypotension

Table 4
Ten guiding facts in the management of OH

Table 5
Some drugs used to treat supine hypertension

Acknowledgements
This work was supported in part by National Institutes of Health (NS 44233 Pathogenesis and Diagnosis of Multiple System Atrophy, U54 NS065736 Autonomic Rare Disease Clinical Consortium), Mayo CTSA (UL1 TR000135), The Kathy Shih Memorial Foundation, and Mayo Funds.
The Autonomic Diseases Consortium is a part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by U54 NS065736 from the National Institute of Neurological Diseases and Stroke (NINDS) and the NIH Office of Rare Diseases Research (ORDR).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
References
1. Low PA. Update on the evaluation, pathogenesis, and management of neurogenic orthostatic hypotension: introduction. Neurology. 1995; 45:4 Suppl 5. S4–S5.
2. Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007; 82:308–313.

3. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996; 46:1470.
5. Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008; 7:451–458.

6. Low PA, Walsh JC, Huang CY, McLeod JG. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain. 1975; 98:341–356.

7. Fujimura J, Camilleri M, Low PA, Novak V, Novak P, Opfer-Gehrking TL. Effect of perturbations and a meal on superior mesenteric artery flow in patients with orthostatic hypotension. J Auton Nerv Syst. 1997; 67:15–23.

8. Lipp A, Sandroni P, Ahlskog JE, Fealey RD, Kimpinski K, Iodice V, et al. Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Arch Neurol. 2009; 66:742–750.

9. Thaisetthawatkul P, Boeve BF, Benarroch EE, Sandroni P, Ferman TJ, Petersen R, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology. 2004; 62:1804–1809.

10. Low PA, Walsh JC, Huang CY, McLeod JG. The sympathetic nervous system in alcoholic neuropathy. A clinical and pathological study. Brain. 1975; 98:357–364.

11. Ketch T, Biaggioni I, Robertson R, Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation. 2002; 105:2518–2523.
12. Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983; 14:573–580.

14. Low PA. Laboratory evaluation of autonomic function. Suppl Clin Neurophysiol. 2004; 57:358–368.
15. Denq JC, O'Brien PC, Low PA. Normative data on phases of the Valsalva maneuver. J Clin Neurophysiol. 1998; 15:535–540.

16. Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989; 64:617–628.

17. El-Sayed H, Hainsworth R. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart. 1996; 75:134–140.

18. Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Midodrine Study Group. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension A randomized, double-blind multicenter study. JAMA. 1997; 277:1046–1051.

19. Figueroa JJ, Basford JR, Low PA. Preventing and treating orthostatic hypotension: as easy as A, B, C. Cleve Clin J Med. 2010; 77:298–306.

20. Smit AA, Wieling W, Fujimura J, Denq JC, Opfer-Gehrking TL, Akarriou M, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res. 2004; 14:167–175.

21. Denq JC, Opfer-Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res. 1997; 7:321–326.

22. Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet. 1999; 353:723.

23. Jordan J, Shannon JR, Black BK, Ali Y, Farley M, Costa F, et al. The pressor response to water drinking in humans: a sympathetic reflex? Circulation. 2000; 101:504–509.
24. Bouvette CM, McPhee BR, Opfer-Gehrking TL, Low PA. Role of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentation. Mayo Clin Proc. 1996; 71:847–853.

25. Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets. 2007; 7:63–70.

26. Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014; 83:328–335.

27. Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, et al. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006; 63:513–518.

28. Shibao C, Gamboa A, Diedrich A, Biaggioni I. Management of hypertension in the setting of autonomic failure: a pathophysiological approach. Hypertension. 2005; 45:469–476.

29. Rodstein M, Zeman FD. Postural blood pressure changes in the elderly. J Chronic Dis. 1957; 6:581–588.

30. Caird FI, Andrews GR, Kennedy RD. Effect of posture on blood pressure in the elderly. Br Heart J. 1973; 35:527–530.

31. Myers MG, Kearns PM, Kennedy DS, Fisher RH. Postural hypotension and diuretic therapy in the elderly. Can Med Assoc J. 1978; 119:581–585.
32. MacLennan WJ, Hall MR, Timothy JI. Postural hypotension in old age: is it a disorder of the nervous system or of blood vessels? Age Ageing. 1980; 9:25–32.

33. Lennox IM, Williams BO. Postural hypotension in the elderly. Clin Exp Gerontol. 1980; 2:313–329.
34. Palmer KT. Studies into postural hypotension in elderly patients. N Z Med J. 1983; 96:43–45.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download