Abstract
Background
Mal de debarquement (MdD) literally means "sickness of disembarkation", and refers to the illusion of movement perceived as an after-effect of traveling on a boat, train, or airplane. The pathophysiology of MdD is currently unknown.
Case Report
A 20-year-old man presented with dizziness and swaying sensation for 3 days after a boat trip. Compared with the follow-up EEG without symptoms, the EEG recorded while having MdD symptoms disclosed a significantly decreased alpha-band current source density at the precentral gyrus of the left frontal lobe and increased beta-2 activity at the parahippocampal gyrus of right mesial temporal region.
Mal de debarquement (MdD) refers to the illusion of movement perceived as an after-effect of transportation.1 The pathophysiology of MdD remains unknown. Considering the occasional reemergence of spontaneous MdD with prolonged symptoms, the associated neuroplasticity, an adaptation process to externally oscillating environments, appears to be stored somewhere in the cerebral cortices and can be kindled or reactivated.1 Using standardized low-resolution brain electromagnetic tomography (sLORETA; The KEY Institute for Brain-Mind Research, Zurich, Switzerland),2 we observed the presence of deranged cortical activity associated with MdD symptoms, which supports the notion that a cortical mechanism is involved in the pathogenesis of MdD.
A 20-year-old man presented with dizziness and swaying sensation for 3 days after a boat trip. He had experienced seasickness or dizziness either during or after the trip, which lasted 2-3 hours. However, he began to feel dizzy and a swaying sensation when he came home after an additional bus trip of about 4 hours. He complained of fatigue and drowsiness during the day. Ear symptoms were denied. He had no recent history of medication and infection. He also denied previous history of ear disease, migraine, seizure, or syncope. His family history
was unremarkable.
An examination produced no abnormal neurological or neurotological findings. Vestibular function tests including vestibular evoked myogenic potential, subjective visual vertical, caloric test, and pure-tone audiometry were normal. Brain MRI and cerebrospinal fluid findings were also normal. Eight days later the patient began to improve without medication, and his symptoms disappeared completely a further 2 days later.
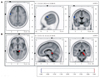
The patient underwent EEG while he suffered from dizziness and disequilibrium (3 days after symptom onset), and had a follow-up EEG 7 days later when the symptoms had disappeared completely. Compared with the follow-up EEG, the EEG while having MdD symptoms indicated a significantly decreased alpha-activity current source density (8-12 Hz) at the precentral gyrus (Brodmann area 6) of the left frontal lobe (Fig. 1A) and increased beta-2 activity (19-21 Hz) at the parahippocampal gyrus (Brodmann areas 27, 30, 35, and 36) of the right mesial temporal region (Fig. 1B). In contrast, the delta, theta, and lower beta bands did not differ significantly between the EEGs.
We observed changes in the cortical activities of a young man with MdD. During the symptomatic period the patient showed decreased alpha activity in the precentral gyrus and increased beta activity in the parahippocampal gyrus of the mesial temporal region. Vestibular inputs project to cortical areas such as the posterior parietal operculum (i.e., human homologue of the parietoinsular vestibular cortex, dorsal aspect of the mesial superior temporal area, and hippocampus.1,3 The mesial superior temporal area is involved in self-motion perception since it receives both visual and vestibular inputs. The hippocampus also plays an important role in spatial memory. Bilateral vestibular loss may lead to spatial memory deficit and even to hippocampal atrophy.4
MdD has been considered a vestibular hallucination. High-frequency activity in the range of 16-32 Hz or beta activity is known to reflect cortical activation that represents an analog of sensory processing, focused attention, or memory.5 Thus, the increased beta activity at the parahippocampal gyrus of the mesial temporal region in our patient supports the assumption that abnormal processing of motion perception can be associated with a hallucination of self-motion in MdD. Interestingly, this feature was observed only on the right side. The right hippocampus is related to allocentric spatial memory and proximity judgments, whereas the left hippocampus is engaged in topokinetic memory. Allocentric perception refers to viewpoint-independent representations of spatial scenes.4 Therefore, overwhelming spatial memory processing - manifested by an increased beta current source in this area - supports the overall phantom phenomenon. The decreased alpha activity in the frontal lobe in our patient might be associated with other symptoms such as fatigue and cognitive slowing in patients with MdD.1 Indeed, our patient complained of fatigue during the symptomatic period. On the other hand, vertigo can be induced by epileptic discharges within the frontal cortex.6 However, the decreased alpha activity in our patient is inconsistent with this explanation.
This study has the limitations of being a single case study and the technical constraints associated with sLORETA analysis. sLORETA cannot generate high-resolution images, and occasionally yields localization errors, particularly when the sources are located deep in the medial and inferior aspects of the brain.7 Therefore, studies using functional imaging may further elucidate this unique disorder.
To the best of our knowledge this is the first study to document the cortical correlates of MdD using an EEG source-localization method.
Figures and Tables
Fig. 1
Voxel-based nonparametric statistical map of sLORETA images. Ten artifact-free, 1.5-s epochs were selected from each EEG recording, made with the subject's eyes closed. Cross-spectra were computed for six frequency bands [delta (1-3 Hz), theta (4-7 Hz), alpha (8-12 Hz), beta-1 (13-18 Hz), beta-2 (19-21 Hz), and beta-3 (22-30 Hz)]. sLORETA was subsequently used to estimate the three-dimensional intracerebral current source density distribution in the frequency domain directly from the average cross-spectral matrix for each frequency band. Voxel-wise statistical nonparametric mapping was used to evaluate differences in sLORETA power between two conditions. Compared with the EEG performed after resolution of the symptoms, the EEG recorded while experiencing MdD symptoms exhibited a significant decrease in alpha activity at the precentral gyrus (Brodmann area 6) of the left frontal lobe (A) and an increase in beta-2 activity at the parahippocampal gyrus (Brodmann areas 27, 30, 35, and 36) of the right mesial temporal region (B). The red color indicates a significant increase and the blue color denotes a significant decrease in current density at a 5% significance level after corrections for multiple comparisons. MdD: Mal de debarquement, sLORETA: standardized low-resolution brain electromagnetic tomography.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090794).
References
2. Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002. 24:Suppl D. 5–12.
3. Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007. 10:1038–1047.

4. Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005. 128:2732–2741.

5. Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001. 5:363–374.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download