Abstract
Background
Cluster headache is a primary headache disorder characterized by periodic episodes of intense headache accompanied by autonomic symptoms. We report an unusual clinical presentation of cluster headache that was preceded by myoclonus and accompanied by hemiparesis.
Case Report
A 26-year-old man visited hospital due to recurrent jerky movements on the left side of his face and neck area lasting 3 days. These jerky movements had disappeared spontaneously without specific treatment. On the 10th day after onset of the jerky movements, the patient developed a series of unilateral severe headaches that were accompanied by autonomic symptoms lasting 1-2 hours. According to the second edition of The International Classification of Headache Disorders, he was diagnosed as having cluster headache. Two of the 16 severe headache attacks this patient suffered were accompanied by dysarthria and hemiparesis. Electroencephalography performed during hemiparesis revealed diffuse lateralized slow activity on the ipsilateral hemisphere of the headache side. The headache and accompanying hemiparesis disappeared after medical treatment for cluster headache.
Cluster headache is a form of 'trigeminal autonomic cephalalgia' with features including severe, unilateral, and retro-orbital pain, restlessness, and parasympathetic autonomic symptoms such as lacrimation or conjunctival injection. Cluster headache usually affects patients aged 20-60 years and has a circadian or circannual periodicity. It occurs is less than 1% of the total population, and is more common in males than in females.1 Cluster headache is characterized by autonomic symptoms, with other neurologic symptoms such as hemiparesis and myoclonus being uncommon. We report an unusual case of cluster headache that was preceded by myoclonus and was associated with hemiparesis.
A 26-year-old healthy man visited our outpatient clinic with presenting symptoms that included recurrent jerky movements, which had been occurring for the previous 3 days. Asynchronous jerky movements appeared in his left face and neck area and lasted approximately 5 minutes, with irregular frequency, over the 3 days before his first visit to the hospital. The jerky movement was observed to be a form of myoclonus. He also complained of an episode of paresthesia that had occurred 10 days previously, starting in his left hand and gradually spreading up and over his left arm, trunk, face, and leg for approximately 2 minutes. He was an office worker with no history of headache, stroke, or seizure. There was no reported family history of headache or other neurologic disorders. A neurological examination revealed no focal neurological deficit between jerky movements. Magnetic resonance imaging and magnetic resonance angiography had normal findings. Cerebrospinal fluid analysis was normal (opening pressure, 15 cmH2O; white blood cell count, 0/mm3; red blood cell count, 0/mm3; protein, 26.4 mg/dL; glucose, 63 mg/dL). Electroencephalography (EEG) findings were normal for both the awake and sleep-deprived conditions. The jerky movements spontaneously disappeared less than 1 day after admission without specific treatment.
The patient revisited the clinic 7 days after discharge, presenting with excruciating headache on the right orbitotemporal area. He had never experienced this type of headache previously. He reported that the headache occurred around the times of sleep onset and waking, and lasted for 1-2 hours. Unilateral autonomic symptoms including lacrimation, rhinorrhea, eyelid edema, and ptosis appeared on the same side as the headache focus. However, symptoms of nausea, vomiting, photophobia, and phonophobia were absent. Thus, we diagnosed this patient as having cluster headache and referred him to the outpatient clinic for commencement of the following medical treatment: verapamil (40 mg tid), valproate (300 mg tid), and zolmitriptan (2.5 mg prn).
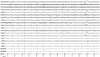
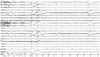
The patient developed a third cluster headache attack, which was associated with dysarthria and hemiparesis. The clinical features of this headache were the same as for the previous two. The hemiparesis was contralateral to the side of the headache, lasted 3 hours, and occurred within 30 minutes of cluster onset. During hemiparesis, the left upper limb was Medical Research Council grade 3 and the left lower limb was grade 4. His deep tendon reflex was normal and the Babinski sign was negative. Repeat brain Magnetic resonance imaging and magnetic resonance angiography revealed no abnormal findings. Echocardiography and laboratory tests such as lipid profile and autoimmune antibodies, including lupus anticoagulant, rheumatoid factor, antinuclear antibody, antineutrophilic cytoplasmic antibody, and anticardiolipin antibody, were normal. Using 24-hour video EEG monitoring, we detected lateralized slow waves on the right hemisphere during a headache accompanied by hemiparesis (Fig. 1). The slow-wave activity normalized after the headache subsided with the aid of oxygen inhalation (Fig. 2). Overall the patient experienced a total of 16 headache attacks over a 4-week period, each of which lasted from 15 minutes to 3 hours, and with a frequency of one to five times per day. The headaches occurred mainly at night (6 p.m. to 8 p.m.) and during the early morning (4 a.m. to 6 a.m.). He experienced daytime headache attacks only occasionally, two of which were accompanied by hemiparesis and lasted for 3 hours and for 30 minutes. During headache attacks, the patient's pain was relieved by oxygen inhalation. In addition, 60 mg of prednisone was prescribed to reduce the severity and frequency of headache, and this proved to be effective.
The patient was discharged without any neurological deficit. He was prescribed verapamil and valproate for 2 months th-rough the outpatient clinic, and prednisone tapered over 5 weeks. He did not complain of further headaches and did not want to continue this treatment due to gastric side effects, so we inst-ructed him in the use of zolmitriptan and oxygen therapy for acute treatment. No headache episode occurred after his discharge.
Prior to the development of typical cluster headache, our patient experienced myoclonus without headache. He then suffered severe, unilateral, orbitotemporal headache accompanied by autonomic symptoms. His headache attacks lasted for up to 3 hours and occurred from once to five times daily. These reported symptoms fulfilled the criteria of the second edition of the International Classification of Headache Disorders for episodic cluster headache.1 Two of the headache episodes were accompanied by transient hemiparesis.
Cluster headache is generally characterized by severe, recurrent, unilateral, periorbital pain and autonomic symptoms. However, hemiparesis rarely presents in cluster headaches.2,3 Aura, which is another neurologic symptom, also rarely presents in these cases. When aura does occur, it can be a visual or olfactory aura.4 Siow et al.2 described four cases of cluster headache with accompanying hemiparesis that was ipsilateral to the pain in two patients and contralateral to the pain in one. The experience of the last patient was similar to that of our patient, with ipsilateral hemiparesis on the face and contralateral hemiparesis on the body. Langedijk et al.3 described a patient who experienced paresthesia that commenced on the right foot and gradually spread up his body over his right trunk, to his right arm and face, with a simultaneous severe, left-sided, retro-orbital stabbing pain. Right hemiparesis appeared a few minutes after paresthesia began, and disappeared after the headache was relieved. These previous reports and our case both demonstrate that the side exhibiting hemiparesis is not always the same as the side exhibiting headache. The reported duration of hemiparesis has ranged from 30 minutes to 24 hours, and the hemiparesis subsided with headache relief. Cluster headache attacks were occasionally associated with hemiparesis, and the clinical features of the headache, such as its pattern, duration, and frequency, did not vary with the occurrence of accompanying hemiparesis. In our case, the cluster headache was accompanied by hemiparesis on 2 out of 16 cluster headache attacks. This hemiparesis started after the onset of the headache and improved with amelioration of the headache. In addition, our case was preceded by a series of myoclonus episodes.
The pathophysiology of cluster headache is generally explained in the context of three main clinical features: trigeminal pain distribution, cranial autonomic features, and an episodic pattern of attack. Pain is caused by activation of the trigeminovascular system, which primarily consists of trigeminal afferents innervating the meningeal blood vessels, the trigeminal nerve, and the brainstem nuclei that modulate sensory signal transmission. Autonomic symptoms are due to activation of the cranial parasympathetic outflow from the cranial nerve.5,6 However, the hemiparesis associated with cluster headaches is not understood within the context of this well-known pathophysiology. Considering the clinical similarity of reversible hemiparesis with familial hemiplegic migraine (FHM), we suggest that the clinical presentation of hemiparesis is caused by transitory electrical silence. In FHM, channelopathy by an identified mutant gene plays a role in neuronal hyperexcitability. Mutation of the α1 subunit of voltage-gated P/Q-type calcium channels (FHM-I), the α2 subunit of Na/K ATPase (FHM-II), or the α1 subunit of voltage-gated sodium channels (FHM-III) all produce the same effects, including an increase in cortical excitability, possibly mediated by high amounts of synaptic glutamate and/or extracellular potassium ions.7,8 This neuronal hyperexcitability triggers cortical spreading depression (CSD), which has been shown to activate the trigeminovascular system.9 Therefore, we suggest that cortical hyperexcitability and CSD induce focal neurologic deficits such as hemiparesis. Some studies have found CSD to be accompanied by flattening EEG activity,10 and EEG studies in hemiplegic migraine cases have demonstrated that severe unilateral or focal disturbances of delta activity, theta activity, theta-delta activity, or alpha-reduction occur during ictal events.11,12 In our case, EEG exhibited diffuse lateral theta-delta slow-wave activity on the ipsilateral hemisphere of the headache side (Fig. 1).
It is unclear why this patient's myoclonus appeared a few days before the headache occurred. Myoclonus has not previously been reported in cluster headache cases. With this observation alone, we did not diagnose our case as a cluster headache until witnessing the headache in a clinical setting. Therefore, this case should remind clinicians to consider that there may be diverse clinical manifestations in conjunction with a cluster headache.
Figures and Tables
References
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004. 24:Suppl 1. 9–160.
2. Siow HC, Young WB, Peres MF, Rozen TD, Silberstein SD. Hemiplegic cluster. Headache. 2002. 42:136–139.

3. Langedijk M, van der Naalt J, Luijckx GJ, De Keyser J. Cluster-like headache aura status. Headache. 2005. 45:80–81.

5. Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. Lancet Neurol. 2002. 1:251–257.

7. Ramagopalan SV, Ramscar NE, Cader MZ. Molecular mechanisms of migraine? J Neurol. 2007. 254:1629–1635.

8. Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008. 65:709–714.

9. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002. 8:136–142.

10. Marshall WH. Spreading cortical depression of Leao. Physiol Rev. 1959. 39:239–279.
11. Sand T. EEG in migraine: a review of the literature. Funct Neurol. 1991. 6:7–22.
12. Varkey B, Varkey L. EEG in hemiplegic migraine. Neurol India. 2004. 52:134.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download