Abstract
Background and Purpose
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited microangiopathy caused by mutations in the Notch3 gene. Although previous studies have shown an association between lacunar infarction and cognitive impairment, the relationship between MRI parameters and cognition remains unclear. In this study we investigated the influence of MRI parameters on cognitive impairment in CADASIL.
Methods
We applied a prospective protocol to 40 patients. MRI analysis included the normalized volume of white-matter hyperintensities (nWMHs), number of lacunes, and number of cerebral microbleeds. Cognition was assessed with the aid of psychometric tests [Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-cognition (ADAS-cog), Trail-Making Test, and Stroop interference (Stroop IF)].
Results
A multivariate regression analysis revealed that the total number of lacunes influenced the performance in the MMSE, ADAS-cog, and Stroop IF, while nWMHs had a strong univariate association with ADAS-cog and Stroop IF scores. However, this association disappeared in the multivariate analysis.
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited angiopathy caused by mutations in the Notch3 gene.1 The characteristic features include recurrent ischemic stroke, migraine, mood disturbances, apathy, and cognitive impairment.2 Cognitive impairment is the second most frequent clinical manifestation, with executive dysfunction and slowness of cognitive processing speed present in most patients.3 The cognitive profile in CADASIL is characteristic of vascular dementia and is similar to that reported for sporadic subcortical ischemic vascular dementia.4
MRI plays a major role in the diagnosis of CADASIL. Typical MRI findings include multiple focal lacunar infarcts and diffuse T2-weighted hyperintensities of the cerebral white matter.5 Recent MRI studies have shown that cognitive impairment is associated with neuroimaging measures in CADASIL, but that there are conflicting results.6-11 Some researchers have observed an association between the number of lacunar infarctions and the severity of cognitive impairment,6-8 while other also found cognitive impairment to be associated with ventricular enlargement,8 brain atrophy,9 and cerebral microbleeds (CMBs).8,10
In the present study we examined several different MRI parameters and determined their effect on cognitive impairment in patients with CADASIL.
Between April 2008 and May 2009, we enrolled 59 consecutive patients who had genetically confirmed CADASIL. Patients who did not undergo a prospective MRI protocol (n=8) or a comprehensive neuropsychological protocol (n=11) were excluded, resulting in the final inclusion of 40 patients in the study. The vascular risk factors were recorded, including hypertension, diabetes mellitus, and hypercholesterolemia. Hypertension was defined as blood pressures of >140/90 mm Hg on different oc-casions or use of an antihypertensive agent. Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL, a PP2 test level of ≥200 mg/dL, or the use of antidiabetes medication. Hypercholesterolemia was defined as a total serum cholesterol level of >240 mg/dL. This study was approved by the institutional review board, and informed consent was obtained from patients.
All patients received a standardized battery of neuropsychological tests. Global cognitive function was assessed with the Mini-Mental State Examination (MMSE)12 and the Alzheimer's Disease Assessment Scale-cognition (ADAS-cog),13,14 which includes tests for orientation, language, ideational and constructional praxis, and memory. Executive function was assessed using the Trail-Making Test part B (TMT-B)15-17 and the color interference section of the Stroop Color and Word test (Stroop IF).18 The neuropsychological examinations were performed by a trained neuropsychologist who was blinded to the patients' imaging results.
MRI scans were obtained with a 1.5-T system (Siemens, Sonata, Germany). The brain imaging protocol, which used a slice thickness of 5 mm and an interslice gap of 1.5 mm, employed the following parameters: T1-weighted images [time to echo (TE)=9.3 ms and time to repeat (TR)=550 ms], T2*-weighted gradient echo planar images (TE=20 ms and TR=600 ms), and fluid attenuated inversion recovery (FLAIR) images (TE=135 ms, TR=8,100 ms, and inversion time=2,100 ms).
Lacunar infarcts were defined as parenchymal defects not extending to the cortical gray matter with a signal intensity of cerebrospinal fluid in all sequences and more than 2 mm in diameter. The total number of lacunar infarctions was counted manu-ally by two observers. Lesions located in the lower third of the corpus striatum of the basal ganglia were excluded.19
CMBs were defined as focal areas of round signal loss on T2*-weighted gradient echo planar images with a diameter of less than 10 mm.20 The total number of CMBs was counted manually by two observers. Areas of symmetric hypointensity in the basal ganglia were excluded.
White-matter hyperintensities (WMHs) were defined as white-matter areas with increased signal intensities on FLAIR images. All FLAIR axial sections from the base of the cerebellum to the vertex were analyzed. A masking and thresholding technique was used (Analyze 8.1, Biomedical Imaging, Mayo Clinic, Rochester, MN, USA) (Fig. 1). The total volume of WMHs was calculated automatically by multiplying the lesion area by the section thickness. WMH volume was normalized for total brain volume by dividing the individual WMH volume by the intracranial cavity volume [normalized WMH volume (nWMH)]. WMH volumes were measured by two investigators who were blinded to the clinical data.
Data were analyzed using SPSS statistical software (version 12.0). The correlation between MMSE, ADAS-cog, and Stroop IF scores, and time to completion of the TMT-B versus MRI parameters (number of lacunes, number of CMBs, and nWMH) were analyzed by Spearman correlation, corrected for potential confounding by age. Linear regression models were used to assess the relative contribution of MRI parameters on clinical scales. Independent variables were the education level, number of lacunes, number of CMBs, and nWMH. We first used univariate regression models, followed by multivariate models. A p value of less than 0.05 was considered to be indicative of statistical significant.
Descriptive data for all participants are reported in Table 1. Twenty-five (62.5%) of the 40 patients were men. The patients were aged 58.1±9.9 years (mean±SD; range, 38-74 years). The sites of the mutations were R544C (n=37), R578C (n=2), and R75P (n=1). Thirty-six subjects were symptomatic and 4 were asymptomatic. Ischemic stroke was the most frequent manifestation (n=20), followed by chronic headache (n=9), dementia (n=3), intracerebral hemorrhage (n=3), and seizure (n=1). Hypertension was present in 19 patients. Other risk factors included diabetes (n=2), hypercholesterolemia (n=7), and history of smoking (n=16). Twenty-four patients (60%) had lacunar infarctions, with 7.0±6.1 (range 1 to 20) per patient. CMBs were found in 21 (52.5%) patients, with 12.0±18.1 (range 1-81) per patient. The nWMH was 3.5±1.9.
The MMSE and ADAS-cog scores were 27.9±2.9 and 11.8±8.0, respectively. After adjustment for age, a significant association was found between the number of lacunes and the scores on all cognitive tests except TMT-B. A univariate analysis (Table 2) revealed an association between the MMSE score and the number of lacunes (p=0.005). Furthermore, the ADAS-cog score was associated with the number of lacunes (p<0.001) and nWMH (p=0.002). The total number of lacunes and nWMH showed trends toward poor performance in the TMT-B (p=0.084 and p=0.083, respectively). The Stroop IF score was associated with the number of lacunes (p<0.001) and nWMH (p=0.019). There was no significant association between the scores on any of the cognitive tests and the number of CMBs. A higher education level was associated with better performance in all cognitive tests except the Stroop IF.
The results of the multivariate regression analysis are summarized in Table 3. The number of lacunes was an independent predictor of cognitive impairment for all cognitive tests in the multivariate analysis. There was also a significant independent effect of education level on all cognitive tests. nWMHs was associated with ADAS-cog and Stroop IF scores. However, the multivariate analysis found no significant association with nWMHs.
We found that the number of lacunes was the most important predictor of cognitive impairment in patients with CADASIL. Of the candidate variables investigated in our study, education level, number of lacunes, and nWMH showed univariate associations with cognitive scales. However, only the number of lacunes and education level remained significant in multivariate modeling. This is consistent with previous studies finding an association between the number of lacunar infarctions and the severity of cognitive impairment.6-9 The association between MRI parameters and clinical scales (cognitive function and disability) has been described previously for Caucasians.6-10 However, no studies have investigated clinical and MRI correlates in Asian patients with CADASIL. This is the first study to demonstrate that the number of lacunes is a major contributor to cognitive impairment in non-Caucasian CADASIL patients.
Our data show that the number of lacunes is negatively correlated with specific executive function as well as global cognitive function. The number of lacunes was independently associated with Stroop IF scores, which were sensitive discriminators between patients with CADASIL and healthy subjects in the early disease stages, as reported previously.3 In addition, lacunar infarcts were independent predictors of global cognitive decline as assessed by MMSE and ADAS-cog. ADAS-cog is one of the most widely used scales for measuring cognitive impairment. However, an association between the number of lacunes and ADAS-cog scores has not been described before in CADASIL.
It is unusual that no significant correlation was found between the number of lacunes and time to completion of the TMT-B in the present study. However, there was a trend toward an association between the number of lacunes and poor performance in the TMT-B (p=0.084). Moreover, a significant association was found between these two parameters after correcting for age and education level (p=0.04, r=0.35).
Recent studies of the relationship between CMBs and cognition have produced conflicting results, with some showing no association between the two,6,7 whereas others indicating a possible association with cognition.8-10 One prospective study found longitudinal associations between CMBs and cognitive decline.8 However, the number of patients in that analysis (n=25) was too small to draw definitive conclusions. Our study found that CMBs were not associated with cognitive scales in the univariate analysis. We cannot exclude the possibility that CMBs contributed to cognitive impairment in CADASIL. However, our results suggest that the impact of CMBs on cognition is less significant than that of the number of lacunar infarctions.
We also found that the association between nWMHs and cognitive scales was present in the univariate analysis, but this association disappeared in the final multivariate model. These findings are in line with previous studies showing the importance of lacunar lesions but not WMH volume in patients with CADASIL.6-9
Our study was subject to several limitations. First, the number of lacunar infarcts and CMBs at each cerebral location were not quantified, and hence we were unable to determine the correlation between regional MRI abnormalities and cognitive scales. Second, we were not able to measure the volume of individual lacunar infarcts and CMBs, which may have affected cognitive deficits. Third, we could not evaluate the degree of brain atrophy, which has been reported to be an important predictor of cognitive impairment. Finally, R544C in exon 11 accounted for 92.5% of the mutations in our study. Thus, our findings may not be fully representative of the wider CADASIL population.21,22
In conclusion, we found that the total number of lacunes was associated with poor performance in the MMSE, ADAS-cog, and Stroop IF. These findings suggest that the number of lacunes can be used as a predictive factor of cognitive impairment in non-Caucasian patients with CADASIL. However, since this was a cross-sectional study, further prospective studies are needed to elucidate the association between MRI parameters and cognitive impairment.
Figures and Tables
Fig. 1
An example of the volumetric analysis. Whole brain volume was calculated after removing nonbrain tissue (B) and the ventricles (C). The volume of the white-matter hyperintensity was calculated semiautomatically using threshold filtering (D).
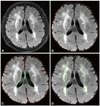
Table 2
Univariate analysis of the effect of MRI parameters on cognitive function

Lacunes: number of lacunar infarcts, beta: standardized beta, ns: not significant at p=0.05.
nWMH: normalized white-matter hyperintensities volume, CMBs: cerebral microbleeds, MMSE: Mini-Mental State Examination, ADAS-cog: Alzheimer's Disease Assessment Scale-cognition, TMT-B: Trail-Making Test part B, Stroop IF: Stroop interference.
Acknowledgements
This work was supported by the research grant of the Jeju National University in 2008.
References
1. Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996. 383:707–710.

2. Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet. 1995. 346:934–939.
3. Peters N, Opherk C, Danek A, Ballard C, Herzog J, Dichgans M. The pattern of cognitive performance in CADASIL: a monogenic condition leading to subcortical ischemic vascular dementia. Am J Psychiatry. 2005. 162:2078–2085.

4. Benisty S, Hernandez K, Viswanathan A, Reyes S, Kurtz A, O'Sullivan M, et al. Diagnostic criteria of vascular dementia in CADASIL. Stroke. 2008. 39:838–844.

5. O'Sullivan M, Jarosz JM, Martin RJ, Deasy N, Powell JF, Markus HS. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001. 56:628–634.
6. Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O'Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007. 69:172–179.

7. Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, et al. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007. 38:923–928.

8. Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009. 72:143–148.
9. Viswanathan A, Godin O, Jouvent E, O'Sullivan M, Gschwendtner A, Peters N, et al. Impact of MRI markers in subcortical vascular dementia: a multi-modal analysis in CADASIL. Neurobiol Aging. 2010. 31:1629–1636.

10. Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006. 129:2375–2383.

11. Jouvent E, Viswanathan A, Mangin JF, O'Sullivan M, Guichard JP, Gschwendtner A, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007. 38:1786–1790.

12. Kang Y, Na DL, Hahn S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.
13. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984. 141:1356–1364.

14. Suh GH, Mohs RC. Development of the Korean version of Alzheimers Disease Assessment Scale (ADAS-K) to assess cognition in dementia. J Korean Geriatr Soc. 2003. 7:269–277.
15. Reitan RM. Trail Making Test. Manual for Administration and Scoring. 1959. Bloomington, IN: Indiana University.
16. Yi H, Chin J, Lee BH, Kang Y, Na DL. Development and validation of Korean version of Trail Making Test for elderly persons. Dement Neurocognitive Disord. 2007. 6:54–66.
17. Madureira S, Verdelho A, Ferro J, Basile AM, Chabriat H, Erkinjuntti T, et al. Development of a neuropsychological battery for the Leukoaraiosis and Disability in the Elderly Study (LADIS): experience and baseline data. Neuroepidemiology. 2006. 27:101–116.

18. Golden C, Freshwater SM. Stroop Color and Word Test. 2002. Wood Dale, IL: Stoelting.
19. Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998. 245:116–122.

20. Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006. 66:165–171.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download