Abstract
Background and Purpose
Recent studies have demonstrated that resveratrol (RSV) reduces the incidence of age-related macular degeneration, Alzheimer's disease (AD), and stroke, while melatonin (MEL) supplementation reduces the progression of the cognitive impairment in AD patients. The purpose of this investigation was to assess whether the co-administration of MEL and RSV exerts synergistic effects on their neuroprotective properties against β-amyloid (Aβ)-induced neuronal death.
Methods
The neuroprotective effects of co-treatment with MEL and RSV on Aβ1-42-induced cell death, was measured by MTT reduction assay. Aβ1-42 caused an increase in intracellular levels of reactive oxygen species (ROS), as assessed by H2-DCF-DA dye, and a reduction of total glutathione (GSH) levels and mitochondrial membrane potential, as assessed using monochlorobimane and rhodamine 123 fluorescence, respectively. Western blotting was used to investigate the intracellular signaling mechanism involved in these synergic effects.
Results
We treated a murine HT22 hippocampal cell line with MEL or RSV alone or with both simultaneously. MEL and RSV alone significantly attenuated ROS production, mitochondrial membrane-potential disruption and the neurotoxicity induced by Aβ1-42. They also restored the Aβ1-42-induced depletion of GSH, back to within its normal range and prevented the Aβ1-42-induced activation of glycogen synthase kinase 3β (GSK3β). However, co-treatment with MEL and RSV did not exert any significant synergistic effects on either the recovery of the Aβ1-42-induced depletion of GSH or on the inhibition of Aβ1-42-induced GSK3β activation. Aβ1-42 treatment increased AMP-activated protein kinase (AMPK) activity, which is associated with subsequent neuronal death. We demonstrated that MEL and RSV treatment inhibited the phosphorylation of AMPK.
Alzheimer's disease (AD) is a progressive and age-related neurodegenerative disorder, that is, characterized clinically by irreversible cognitive dysfunction, memory loss, and behavioral changes.1 These features are accompanied by specific pathologic changes in the brain, which manifest as the extracellular deposition of a fibrillar form of amyloid-β1-42 peptide (Aβ1-42) constituted senile plaques. Aβ1-42 is the cleavage fragment made by proteinases from the amyloid precursor protein, which exerts neurotoxic effects concerned with neuroinflammation, immune activation, and oxidative stress and thus has been considered to play a critical role in the pathogenesis of AD.2 However, the mechanism underlying this β-amyloid peptide (Aβ) neurotoxicity remains to be fully elucidated. There is increasing evidence that Aβ alters Ca2+ homeostasis, mitochondrial dysfunction, and apoptosis, and increases the intracellular level of reactive oxygen species (ROS) in the AD brain.
It is well known that the neuronal cell death induced by Aβ1-42 is effected via several pathogenic mechanisms, such as the induction of glycogen synthase kinase-3β (GSK3β).3 There are several recent lines of evidence that neurodegeneration, such as in AD and stroke, is associated with the activation of AMP-activated protein kinase (AMPK).4 However, the signal mechanisms underlying Aβ neurotoxicity have not been elucidated. Thus, in this investigation, we explored the relationship between GSK3β and AMPK signaling in Aβ1-42-induced neurotoxicity.
One of the potential candidates, that can regulate the Aβ-in-duced neuroinflammatory response, is melatonin (N-acetyl-5-methoxy-tryptamine, MEL), which is the main secretory product of the pineal gland,5 and participates in various physiological functions including the control of seasonal reproduction, regulation of circadian rhythms and body temperature via receptors,6 and acts a potent free-radical scavenger and antioxidant.7 The special features of MEL include its role in the regulation of the immune response and exertion of cytoprotective properties in various neurodegeneration models, both in vivo and in cell cultures.8,9 It was recently suggested that MEL plays a critical role as an antioxidant and neuroprotector in aging and AD. Levels of MEL decrease with age, and patients with AD experience an even greater reduction in this hormone. Clinical reports indicate that MEL supplementation improves sleep and slows down the progression of cognitive impairment in patients with AD. It also effectively prevents Aβ-mediated neuronal cell death via its antioxidant and anti-amyloid properties. Interest in MEL has grown following studies in which it was established that 1) MEL can easily cross the bloodbrain barrier;10 2) the endogenous production of MEL falls dramatically with increasing age;11 3) high doses of MEL do not present any harmful side-effects;12 and 4) MEL acts as a free-radical scavenger in most tissues.13 On the basis of these data, MEL has been proposed as a preventive treatment against neurodegenerative disorders; however, the protective mechanism underlying its actions remains to be elucidated.
Another plausible and wide-studied candidate is resveratrol (trans-3,4,5'-Trihydroxystilbene, RSV), which is a natural polyphenolic compound found mainly in the skin of grapes and nuts, pomegranates, and Polygonum cuspidatum, a component of Chinese herbal medicines, and is present at high levels in red wine. RSV is a competent estrogenic product, exhibiting protective effects in cardiovascular diseases, cancer, and neurodegenerative diseases,14 partly as a result of its anti-oxidative, anti-inflammatory, and anti-mutagenic activities.15 There is evidence for beneficial effects of RSV in neurodegenerative conditions, such as cerebral ischemia,16 Parkinson's disease (PD),17 AD,18 and normal aging.19 The action of RSV manifests as the regulation of the activities and expression levels of enzymes and proteins associated with the survival signal, regulation of ion channels, and anti-oxidative actions.20 RSV also activates the sirtuin categorized class III histone deacetylases. Sirtuins play a key role in various cellular processes, including lifespan extension in response to caloric restriction (CR).21,22 However, the mechanisms involved in these protective effects of RSV on neurons are not fully understood.
Based on these observations, we investigated whether combination with MEL and RSV can attenuate Aβ1-42-induced neuronal death. In particular, we focused on the effects of combined treatment on oxidativestress-induced parameters, such as mitochondrial membrane potential (Δψm), GSK3β, and AMPK signaling associated with energy homeostasis and cell survival.
Pregnant Sprague-Dawley (SD) rats were obtained from Orient Bio (Seoul, Korea) and minimum essential medium (MEM) was purchased from JBI (Seoul, Korea). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, and antibiotics were purchased from Gibco (Gaithersburg, MD, USA), and Aβ1-42 peptide was purchased from BACHEM (Bubendorf, Switzerland). RSV, N-acetyl cysteine (NAC), 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), adenine 9-β-D-arabinofuranoside (Ara-A), and MEL were purchased from Sigma Chemical (St. Louis, MO, USA). PD98059 (PD) and SB203580 (SB) were purchased from Calbiochem, EMD Biosciences (La Jolla, CA, USA), and SP600125 (SP) and Trolox were purchased from Biomol (Plymouth Meeting, PA, USA). Monochlorobimane (mBCl) was purchased from Fluka (St. Louis MO, USA) and H2-2'7'dichlorofluorescein diacetate dye (H2DCF-DA), dihydroethidium (DHE), and rhodamine 123 were purchased from Molecular Probes (Eugene, OR, USA).
All experimental procedures were carried out using protocols approved by the Institutional Animal Care and Use Committee of Konkuk University. Murine HT22 hippocampal cells were cultured with DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells were rinsed twice with serum-free medium and then detached with 0.2% trypsin with ethylenediaminetetraacetic acid, and replated at a low density (5,000 cells/cm2) in a 24-well or 6-well plate (Becton-Dickinson, Franklin Lakes, NJ, USA). The re-plated cells were incubated for 24-h before the experiment.
Primary hippocampal neuronal cultures were obtained from the dissociated embryonic day-18 hippocampus of pregnant SD rats. Briefly, the hippocampus, freed of meninges, was mechanically dissociated and gently triturated thrice with a flame-polished Pasteur pipette in the culture medium (Eagle's MEM supplemented with 20 mM glucose). The cells were seeded onto plates coated with 100 µg/mL poly-D-lysine and 200µg/mL laminin in the culture medium supplemented with 5% fetal bovine serum, 5% horse serum, and 2mM glutamine. The cultures were maintained at 37℃ in a humidified incubator containing 5% CO2. For pure neuron cultures, 5 µM cytosine-β-arabinofuranoside was added after 3 days. The cultured cells were used after 7days, by which time the neurons had differentiated from neuronal precursor cells.
Aβ1-42 was dissolved to a 10-mM stock solution in dimethylsulfoxide (DMSO, Sigma Chemical, SIGMA, St. Louis, USA) and diluted in phosphate-buffered saline (PBS) to a final Aβ concentration of 1 mM. Aggregation of the Aβ1-42 peptide was achieved by incubating the cells with the diluted Aβ for 3-h at 37℃, as described previously.23
MEL and RSV were dissolved in ethanol and DMSO, respectively, at final ethanol and DMSO concentrations of 0.1% and 0.01%, respectively. The effects of MEL and RSV on neuronal cell death were determined by evaluating the following: 1) viability of the cells, assessed using a 3-(4,5-dimethylthiazxol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay; 2) measurement of intracellular ROS; 3) measurement of total glutathione (GSH); 4) change in the Δψm; and, 5) activation of the intracellular signaling molecules, such as mitogen-activated protein kinase (MAPKs), GSK3β, and AMPK. The concentration-dependency of the effects of MEL and RSV on Aβ1-42-induced neuronal death was evaluated in HT22 hippocampal cells, incubated for 24-h and 48-h with various concentrations of MEL (1, 10, 50, 100, and 500 µM) and RSV (0.1, 1, 5, 10, and 20 µM).
Cells were pretreated with MEL for 1-h before Aβ1-42, and RSV was added along with Aβ1-42. To establish the participation of AMPK in the neuroprotective effects of MEL and RSV, the cells were incubated for 1-h with the AMPK activator; AICAR (10, 100, or 1,000 µM) or AMPK inhibitor; Ara-A (10, 100, or 1,000 µM), followed by incubation with Aβ1-42, MEL, and RSV for 24-h.
Cell viability was also assessed by the MTT assay. MTT is a water-soluble tetrazolium salt that is reduced by metabolically viable cells to a colored, water-insoluble formazan salt. MTT (5mg/mL) was added to the cell-culture medium. After incubating the plates at 37℃ for 2-h in a 5% CO2 atmosphere, the assay was suspended and the MTT-containing medium was replaced with DMSO. The absorbance was read at 570 nm with a microplate reader (Molecular Devices, Palo Alto, CA, USA). The percentage of surviving neurons was measured relative to control values (untreated cells, 100%).
Intracellular ROS formation was measured by fluorescence using H2DCF-DA.24 This non-fluorescent dye freely permeates into cells, where it de-esterifies to form the ionizedfree acid (dichlorofluorescein), which reacts with ROS to form the fluorescent 2',7'-dichlorofluorescein (DCF). After the drug treatment, cultures were washed with Hank's balanced aqueous salts solution (HBSS) containing 120mM NaCl, 5 mM KCl, 1.6mM MgCl2, 2.3mM CaCl2, 15mM glucose, 20 mM HEPES, and 10 mM NaOH, loaded with 20µM H2DCF-DA and 20% Pluronic F-127 for 30min at 37℃, and then washed again with HBSS and kept at room temperature for an additional 30 min to allow for the complete de-esterification of the dye.
DCF fluorescence was analyzed using a fluorescence plate reader (Spectramax Gemini EM, Molecular Devices) at excitation and emission wavelengths of 490 and 530 nm, respectively, with a fluorescence microscope.
Changes in Δψm were estimated by the uptake of a cell-permeant, lipophilic, cationic, fluorescent dye, rhodamine 123, which enters the mitochondria as a result of the highly negative Δψm. Depolarization of Δψm results in the loss of rhodamine 123 from the mitochondria, resulting in a decrease in the intracellular fluorescence. Treated cells were incubated with rhodamine 123 (10µM) at 37℃ for 20 min, after which they were washed twice with PBS and then observed under a fluorescence microscope (LSM10, Carl Zeiss, Dublin, CA, USA).
To measure the total GSH content, the cells cultured in six-well plates were first washed with PBS, and 20 µM mBCl with PBS was added and incubated for 20 min at 37℃, and then washed again with PBS. Fluorescence was monitored at excitation and emission wavelengths of 390 and 480 nm, respectively, using a fluorescence plate reader (Spectramax Gemini EM, Molecular Devices).
After treatment with Aβ1-42, MEL, and RSV for a specific time period, the cells were harvested and homogenized in 100 µL/well sodium dodecylsulfate (SDS) sample buffer containing 62.5mM Tris-HCl (pH6.8), 2% (w/v) SDS, 10% glycerol, 50 mM dithiothreitol, 0.1% (w/v) bromophenol blue, and 1 mM sodium orthovanadate. After boiling for 5 min, equal amounts of protein were subjected to 10% SDSpolyacrylamide gel electrophoresis for 140 min, after which the separated proteins were transferred electrophoretically to nitrocellulose membranes (Whatman, Dassel, Germany) for 20 min. The blot was blocked with 5% non-fat, dried milk at room temperature and subsequently incubated overnight at 4℃ with the following antibodies: anti-p38, anti-p44/42, anti-cJunNterminal kinases (JNKs), anti-GSK3β, anti-AMPKα, anti-phospho-p38 (Thr180/Tyr182), anti-phospho-p44/42 (Thr202/Tyr204), anti-phospho-JNK (Thr183/Tyr185), anti-phospho-GSK3β (Ser9), anti-phospho-AMPKα (Thr172) (1:1,000), and anti-β-actin. After incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1-h, the bands were detected with an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ, USA) and analyzed using an LAS-3000 image detection system (Fuji, Tokyo, Japan).
We investigated the synergistic effects of combined treatment with MEL and RSV against Aβ1-42-induced neurotoxicity, using an HT22 hippocampal neuronal cell line, primary hippocampal neurons, and an MTT assay. Aβ1-42 treatment induced neuronal cell death in a concentration-dependent manner (data not shown). We tested the protective effects of MEL and RSV at a fixed Aβ1-42 concentration of 2 µM. The concentration ranges of MEL and RSV were 1-500 µM and 0.1-20 µM, respectively. MEL and RSV prevented Aβ1-42-induced neurotoxicity in HT22 hippocampal neuronal cell line in a concentration-dependent manner (Fig. 1A and B). The protective effects of MEL and RSV were also observed in rat primary hippocampal neurons (data not shown). Maximum protection against Aβ1-42-induced neurotoxicity was achieved with 500 µM of MEL and 20 µM of RSV. Low concentrations of MEL (1-10 µM) and RSV (0.1-1 µM) did not prevent Aβ1-42-induced cytotoxicity.
However, co-treatment with MEL and RSV at concentrations that when treated via monotherapy provided no protection synergistically prevented Aβ1-42-induced cytotoxicity (Fig. 1C). These results suggest that cotreatment with MEL and RSV is more effective in preventing Aβ1-42induced cytotoxicity than either MEL or RSV alone.
It is relatively well known that the toxicity of Aβ1-42 is mediated through the production of ROS and oxidative stress-induced damage. Hence, we investigated whether the neuroprotective effects of MEL and RSV blocked Aβ1-42-induced oxidative stress and increased the anti-oxidative properties in the hippocampal neuronal cells. Cellular oxidative stress was determined spectrophotometrically with excitation at 490nm and emission at 530nm emission, based on ROS-mediated conversion of DCFH to fluorescent DCF. Aβ1-42 at about 2 µM increased the intracellular ROS production by about 1.8-folds after 6-h in the hippocampal neuronal cells. MEL and RSV at the testedconcentrations profoundly reduced the Aβ1-42-induced production of ROS to control levels (Fig. 2A) without affecting basal levels of ROS production. Trolox (100 µM, a watersoluble vitamin E analog), which was used as a positive control, reduced the Aβ1-42 induced increase in ROS by about 40%. This indicates that MEL and RSV have potent antioxidant properties even compared with Trolox in Aβ1-42-stimulated HT22 hippocampal cells. In this study, even co-treatment with MEL and RSV did not induce a reduction of the ROS production below control levels, suggesting that MEL and RSV do not perturb the normal range of ROS production, or even probably ROS-mediated physiological intracellular signaling. The Aβ1-42induced increase in ROS as measured by DHE was blocked by cotreatment with MEL and RSV (data not shown).
It has been suggested that the antioxidative properties of MEL and RSV are mediated by the prevention of GSH depletion. The effects of MEL and RSV on the level of GSH, an essential endogenous antioxidant, were examined by studying the effects of MEL and RSV on Aβ1-42-induced GSH depletion. After 12-h of Aβ1-42 treatment, mBCl was applied to the medium and the total GSH levels were determined spectrophotometrically with excitation and emission wavelengths of 390 and 485 nm, respectively. In the hippocampal neuronal cells, treatment with Aβ1-42 led to a decrease of about 20% in intracellular GSH levels within 12-h. Treatment with MEL and RSV restored that GSH depletion to at least the control levels (Fig. 2B). As a result of ROS production, either MEL or RSV was sufficient to prevent GSH depletion; cotreatment with MEL and RSV did not further increase intracellular GSH levels.
It has been reported that both MEL and RSV, prevent mitochondrial oxidative stress. Thus, we examined the effects of MEL, RSV, and a combination of both on Δψm as one of the markers of mitochondrial function. Δψm was assessed using rhodamine 123 fluorescence dye. Aβ1-42 treatment induced a loss of about 20% of Δψm at 6 h, MEL and RSV prevented that loss (Fig. 2C and D). MEL and RSV alone and cotreatment with MEL and RSV together did not elevate Δψm over control values.
Oxidative stress is one of the major stimuli for MAPK signaling cascades, such as extracellular signal related kinase (ERK) 1/2 and p38. It has been shown that Aβ1-42 treatment leads to activation of ERK1/2, which is related to cell death. The effects of MEL and RSV on the MAPK activation induced by Aβ1-42, the cell lysates, with Aβ1-42 peptide with or without MEL and RSV for 30 min. The results were analyzed by Western blotting using anti-phospho-ERK1/2 (Thr202/Tyr204), anti-phospho-p38 (Thr180/Tyr182), and anti-phospho-JNK (Thr183/Tyr185) antibodies. Aβ1-42 treatment resulted in an increase in phosphorylated ERK1/2 and p38 MAPK; this activation was prevented by MEL and RSV in hippocampal neu-ronal cells (Fig. 3A).
Combination treatment with MEL and RSV inhibited p38 phosphorylation, but did not prevent ERK activation. Aβ1-42-induced neurotoxicity was inhibited by 20 µM PD and 20µM SB, which are inhibitors of ERK and p38 MAPK, respectively. Activation of ERK was blocked by its inhibitor, 20 µM PD, and phosphorylation of p38 MAPK was prevented by the inhibitor of p38 MAPK, 20µM SB (Fig. 3B), but not by a JNK inhibitor, SP. These results provide further evidence that ERK1/2 and p38 MAPK are associated with cell death in the presence of Aβ1-42. Moreover, our data demonstrate that the neuroprotective effects of MEL and RSV are mediated through the inhibition of ERK1/2 and p38 activity, and that the inhibitory effects of combination treatment with MEL and RSV on Aβ1-42-induced neurotoxicity are, at least partially regulated, by p38 MAPK.
Activation of GSK3β (Ser9) has been shown to be a key component in the signaling pathways that underlie neurodegenerative diseases. It is presumed that Aβ1-42 peptides are involved in the activation of GSK3β. Conversely, inactivation of GSK 3β by phosphoinositide 3-kinase (PI3K)/Akt is an important neuroprotective mechanism. Hence, we examined whether MEL, RSV, and cotreatment with both induced inactivation of GSK 3β. Aβ1-42 activated GSK3β, which could be prevented by MEL and RSV at 3h after Aβ1-42 treatment (Fig. 4A). Either MEL or RSV alone was sufficient to inactivate GSK3β; combination treatment with MEL and RSV did not show further inhibition of GSK3β activation. Aβ1-42-induced neuronal death was inhibited by treatment with LiCl (100and, 1,000 µM), an inhibitor of GSK3β (Fig. 4B). These results suggest that the neuroprotective effects of MEL and RSV are mediated through an increase in inhibitory GSK3β phosphorylation.
AMPK, a master sensor of energy balance in the peripheral tissues, is phosphorylated and activated when the energy balance is low. RSV exerts multiple beneficial effects similar to those associated with CR, which improves neuronal health; AMPK is thought to be activated during CR. The neuroprotective properties of MEL and RSV led us to hypothesize that the neuronal activation of AMPK is an important component of MEL and RSV activity. We therefore investigated whether AMPK was activated in HT22 hippocampal neuronal cells after Aβ1-42 treatment and whether MEL or RSV alone or their cotreatment inhibited AMPK activation. We found a robust increase in the pAMPK (Thr172) level 24-h after Aβ1-42 treatment (Fig. 5A); that elevation was maintained for 48h. This phosphorylation of AMPK was blocked by treatment with either MEL or RSV; the inhibitory effects of MEL and RSV were augmented by their cotreatment. Ara-A (100 µM, a pharmacological inhibitor of AMPK) prevented Aβ1-42-induced neurotoxicity in HT22 hippocampal neuronal cells (Fig. 5B), and the AMPK-activating compound AICAR (100 µM) induced neuronal cell death in HT22 hippocampal cells (Fig. 5C). These findings demonstrate that neuronal inactivation of AMPK by MEL and RSV could affect neuronal energy homeostasis and contribute to the neuroprotective effects of MEL and RSV.
The reported results show that MEL and RSV prevent the activation of ERK, decrease ROS production, and restore GSH levels, and eventually attenuate neuronal cell death in hippocampal neuronal cells. However, although combination therapy with MEL and RSV did not inhibit the activation of ERK, the production of ROS, or the depletion of GSH, it did exert a synergistic effect in reducing Aβ1-42-induced neurotoxicity. Aβ1-42 reduced the Δψm in the hippocampal neuronal cells, an action that was restored by cotreatment with MEL and RSV. The Aβ1-42 induced potentiation of GSK3β activity was inhibited by, either MEL or RSV alone. Similarly, the Aβ1-42-induced activation of AMPK, a well-known regulator of energy homeostasis, was effectively prevented by treatment with either MEL or RSV. Importantly, combined treatment with MEL and RSV exerted a synergic reduction in AMPK activation.
The aggregation of soluble Aβ1-42 into oligomers/fibrils is one of the key pathological features of AD. We used Aβ1-42 of oligomers/fibrils made by incubation for 3 h at 37℃, as described in the Materials and Methods. Although some batchtobatch variation was observed, the manufactured Aβ1-42 induced neurotoxicity at a rate of 20-40% in HT22 hippocampal cells. Since MEL and its metabolites act as radical scavengesr, they can stimulate the activity of the mitochondrialenzyme (NADH-coenzyme Q reductase, and cytochrome C oxidase) associated with oxidative phosphorylation,25 and subsequently increase ATP production. RSV, a polyphenolic compound, is known to be a potent activator of the sirtuin pathway and mimics the CR, both of which are associated with life-span expansion and aging. As a sirtuin activator, RSV is associated with the induction of genes for oxidative phosphorylation and mitochondrial biogenesis,26 and can stimulate the preservation of brain mitochondrial function after hypoxia-reperfusion. We expected that co-treatment with MEL and RSV would be more protective against Aβ-induced neurotoxicity than either treatment alone. Our data indicate that the neuroprotective effects of MEL and RSV are mediated via a decrease in ROS and an upregulation of the antioxidant system, and that combination treatment with these two factors has a synergic effect on neuroprotection. Therefore, the actions of combined treatment with MEL and RSV could be beneficial for energy preservation and prevention of neuronal cell death.
Combination treatment is a method of treating disease through the simultaneous use of a variety of drugs to eliminate or control the biochemical cause of the disease. The rationale for combination treatment is to use drugs that work via different mechanisms of action without intolerable side effects. However, there have been few studies on combination treatments. It was recently reported that combination therapy may be better than monotherapy in bipolar disorder in patients with bipolar disorder needing long-term treatment, the combination of lithium plus valproate is more effective than valproate alone.27 In the present study, we investigated the effects of combination treatment with MEL and RSV, which are well-known neuroprotectors.
Functionally, RSV exhibits histone deacetylase activity as a sirtuin activator (SIRT1), which is associated with CR and energy preservation, and so could have neuroprotective qualities and promote longevity. SIRT1 is a class III histone deacetylase and is essential for maintaining silent chromatin through the deacetylation of histones. MEL also is known to be an inducer of SIRT1.28 It is possible that a combination of MEL and RSV exerts a synergic effect on SIRT1 activity, and that such combination treatment could thus induce a synergic effect on neuroprotection. Further study is needed to elucidate the exact mechanism underlying this potential effect.
Our results show that ERK activation is related to Aβ1-42-induced neurotoxicity in HT22 hippocampal neurons. Previous studies have shown that ERK activation is associated with cell survival and synaptic sprouting. However, it has been reported recently that Aβ-induced neurotoxicity is regulated by the inhibition of ERK activation in rat organotypic hippocampal slice cultures,29 and that glutamate-induced toxicity is related to ERK activation.30 Although cotreatment with MEL and RSV did not exert a synergistic effect in reducing ERK activation, our results demonstrate that Aβ1-42induced neurotoxicity could be mediated through ERK activation.
AMPK has emerged as a master sensor of energy balance, and is phosphorylated and activated when energy balance is low. In other words, AMPK is activated by increasing the cellular AMP: ATP ratio, whereupon it functions to help preserve cellular energy.4 RSV is associated with CR, increasing the lifespan, and delaying the onset of diseases associated with aging. CR improves neuronal health, and a key enzyme thought to be activated during CR is AMPK. Recent reports suggest that AMPK is highly expressed in cortical and hippocampal neurons under both normal and ischemic conditions. Pharmacological inhibition of AMPK reduces stroke damage, whereas the activation of AMPK by AICAR exacerbates that damage. We hypothesized that AMPK activation is an important mediator of MEL and RSV actions in hippocampal neuronal cells. Our results suggest that MEL and RSV manipulate the neuronal energy balance during Aβ1-42 treatment.
It was shown recently, that RSV activates AMPK signaling and that this activation of AMPK lowers extracellular Aβ accumulation.31 However, MEL did not influence AMPK activation in C2C12 murine skeletal muscle cells.32 Our results show that the Aβ1-42-induced activation of AMPK was inhibited by both monotherapy with either MEL or RSV and by their co-treatment in the murine HT22 hippocampal cell line. Neuronal damage induced by Aβ1-42 activates AMPK, and combination treatment with MEL and RSV synergistically prevented neuronal death by inhibiting the activation of AMPK. However, this effect on AMPK activation needs to be studied further.
In this study, MEL and RSV reversed the Aβ1-42 induced alteration in GSK3β, ERK, and AMPK activity. One obvious question pertains to the interrelationship of the activities of these signaling molecules. For example, activation of the PI3K-Akt-GSK3β pathway can regulate the AMPK pathway, and we reported that inhibition of GSK3β can activate ERK1/2 in cultured rat astrocytes.33 It is clear that understanding the nature of the extensive interplay between these diverse signaling systems may help us to understand the mechanism underlying Aβ-dependent neurotoxicity as well as the protective mechanism induced by MEL and RSV.
In conclusion, our data demonstrate clearly that MEL and RSV have strong neuroprotective properties against Aβ1-42-induced cytotoxicity in HT22 hippocampal neuronal cells, by attenuating oxidative stress through the induction of antioxidants, such as GSH, and the inhibition of GSK3β activity. We also found that combined treatment with MEL and RSV exerts beneficial effects on neuronal survival by inhibiting the activation of AMPK. Our data suggest that cotreatment with MEL and RSV provides a useful therapeutic strategy against Aβ1-42-induced neurotoxicity.
Figures and Tables
Fig. 1
Protective effects of MEL and RSV on Aβ1-42-induced cytotoxicity in HT22 hippocampal neuronal cells. A and B: HT22 cells were treated with the indicated concentrations of MEL (1, 10, 50, 100, and 500 µM) and RSV (0.1, 1, 10, and 20 µM) in the absence or presence of Aβ1-42 for 24h at 37℃. The viability of HT22 cells was determined by the MTT reduction assay after treatment with MEL, RSV, and Aβ1-42. MEL was administered 1-hr before Aβ1-42 treatment, while RSV was added to the medium along with Aβ1-42. C: Cotreatment effects of MEL (1 and 10 µM) and RSV (0.1 and 1 µM). Data are expressed as the mean±S.E.M values of three experiments. The data shown are representative of five independent experiments that gave similar results. **p<0.01 vs. control, †p<0.05, ‡p<0.01 vs. Aβ1-42 alone. MEL: melatonin, RSV: resveratrol, Aβ: β-amyloid.

Fig. 2
Antioxidative properties of MEL and RSV on Aβ1-42-induced oxidative stress in HT22 hippocampal neuronal cells. MEL was administered 1-h before Aβ1-42 treatment, while RSV was added to the medium along with the Aβ1-42. A: Effects of MEL and RSV on Aβ1-42-induced increase in DCF fluorescence. RSV at the indicated concentration was applied concomitantly with 1 µM Aβ1-42 for 6-h in HT22 cells. Intracellular ROS was evaluated by measuring H2DCF-DA fluorescence (490/530 nm), as described in the Materials and Methods. B: Effects of MEL and RSV on Aβ1-42-induced depletion of GSH. After 12-h of Aβ1-42 treatment, mBCl was applied to the medium and total GSH levels were determined spectrophotometrically at 390/480 nm. C: Effects of MEL and RSV on Δψm, as assessed by fluorescence microscopy using the fluorescence dye rhodamine 123, as described in the Materials and Methods. D: Measurement of Δψm by fluorospectrophotometry: MEL (100 µM), RSV (20 µM), M10R1 (MEL 10 µM+RSV 1 µM), and Tro (Trolox, 100 µM). Data are expressed as mean±S.E.M values. The data shown are representative of five independent experiments that gave similar results. *p<0.05, **p<0.01 vs. control, †p<0.05, ‡p<0.01 vs. Aβ1-42 alone. MEL: melatonin, RSV: resveratrol, Aβ: β-amyloid, DCF: dichlorofluorescein.
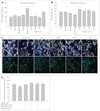
Fig. 3
Effects of MEL and RSV on Aβ1-42-induced MAPK signaling. A: HT22 cells were incubated with 1-µM Aβ1-42 for 30-min in the presence or absence of MEL and RSV at the indicated concentrations, and then harvested for Western blot analysis. Immunoblots of lysates from treated HT22 cells were probed with phospho-ERK (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), and phospho-JNK (Thr183/Tyr185) antibodies. As a loading control, total ERK/p38/JNK levels were also measured (lower panel). MEL and RSV attenuated Aβ1-42-induced activation of ERK and p38 MAPK. B: HT22 cells were treated with the indicated concentrations of PD (inhibitor of ERK), SB (inhibitor of p38 MAPK), and SP (inhibitor of JNK) in the presence of Aβ1-42 for 24-h at 37℃. The viability of HT22 cells was determined by the MTT reduction assay after treatment with PD, SB, and SP with Aβ1-42. Treatment with these MAPK inhibitors blocked the Aβ1-42-induced activation of ERK/p38 MAPK. Data are expressed as the mean±S.E.M values of four independent experiments. **p<0.01 vs. control, †p<0.05, ‡p<0.01 vs. Aβ1-42 alone. MEL: melatonin, RSV: resveratrol, Aβ: β-amyloid, MAPK: mitogen-activated protein kinase, ERK: extracellular signal related kinase.
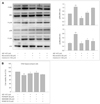
Fig. 4
Inhibitory effects of MEL and RSV on Aβ1-42-induced GSK-3β activation. A: HT22 cells were incubated with 2 µM Aβ1-42 for 6-h in the presence or absence of the indicated concentrations of MEL and RSV, and harvested for Western blot analysis. Immunoblots of lysates from treated HT22 cells were probed with phospho-GSK3β (Ser9) and GSK3β antibodies. Actin levels were measured for the confirmation of equal amount of protein loading (lower panel). MEL and RSV inhibited the Aβ1-42-induced activation of GSK-3β. B: HT22 cells were treated with indicated concentrations of LiCl (inhibitor of GSK3β) in the presence of Aβ1-42 for 24-h at 37℃. The viability of HT22 cells was determined by the MTT reduction assay after treatment of LiCl with Aβ1-42. Data are expressed as the mean±S.E.M values of four independent experiments. **p<0.01 vs. control, †p<0.05, ‡p<0.01 vs. Aβ1-42 alone; RSV (20 µM), MEL (100 µM), MR (MEL 10 µM+RSV 1 µM), Li (LiCl, 1mM). MEL: melatonin, RSV: resveratrol, Aβ: β-amyloid.

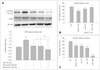
Fig. 5
Effects of MEL and RSV on the activation of AMPK signaling. A: HT22 cells were incubated with 2 µM Aβ1-42 for 24-h in the presence or absence of the indicated concentrations of MEL and RSV, and then harvested for Western blot analysis. Immunoblots of lysates from treated HT22 cells were probed with phospho-AMPK (Thr172) and total AMPK antibodies. Actin levels were also measured to confirm equal amounts of protein loading (lower panel). MEL and RSV inhibited the Aβ1-42-induced activation of AMPK. B: HT22 cells were treated with Ara-A (inhibitor of AMPK) indicated concentrations, and Aβ1-42, or (C) the cells were treated with AICAR (activator of AMPK) without Aβ1-42 for 24-h at 37℃ for the MTT assay. The viability of HT22 cells was determined by the MTT reduction assay. The inhibitor of AMPK blocked Aβ1-42-induced neuronal cell death and the activator of AMPK augmented Aβ1-42-induced neuronal cell death. Data are expressed as the mean±S.E.M values of three independent experiments. **p<0.01 vs. control, †p<0.05, ‡p<0.01 vs. Aβ1-42 alone, §p<0.01 vs. drug only; RSV (20 µM), MEL (100µM), MR (MEL 10 µM+RSV 1 µM), AIC (AICAR, 10-100 µM), Ara-A (10-100 µM). MEL: melatonin, RSV: resveratrol, Aβ: β-amyloid, ERK: extracellular signal related kinase, AMPK: AMP-activated protein kinase.
Acknowledgements
This work was supported by WCU (World Class University) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R33-2008-000-10090-0).
References
1. Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007. 25:577–609.

2. Selkoe DJ. The molecular pathology of Alzhiemer's disease. Neuron. 1991. 6:487–498.
3. Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004. 89:1313–1317.

4. Williams KW, Coppari R, Elmquist JK. "AMPing up" our understanding of the hypothalamic control of energy balance. J Clin Invest. 2007. 117:2089–2092.

5. Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991. 12:151–180.

6. Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006. 41:297–305.

7. Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept. 2000. 9:160–171.

8. Antolín I, Mayo JC, Sainz RM, del Brío Mde L, Herrera F, Martín V, et al. Protective effect of melatonin in a chronic experimental model of Parkinson's disease. Brain Res. 2002. 943:163–173.

9. Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. 2002. 32:163–167.

10. Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995. 19:123–126.

11. Pierpaoli W, Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci U S A. 1994. 91:787–791.

12. Reiter RJ, Pablos MI, Agapito TT, Guerrero JM. Melatonin in the context of the free radical theory of aging. Ann N Y Acad Sci. 1996. 786:362–378.

13. Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, et al. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995. 18:1–11.

14. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. 5:493–506.

15. Zhuang H, Kim YS, Koehler RC, Doré S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann N Y Acad Sci. 2003. 993:276–286.

16. Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006. 78:2564–2570.

17. Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007. 73:550–560.

18. Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005. 280:37377–37382.
19. Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006. 16:296–300.

20. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006. 5:493–506.

21. Han SH. Potential role of sirtuin as a therapeutic target for neurodegenerative diseases. J Clin Neurol. 2009. 5:120–125.

22. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.

23. Mook-Jung I, Joo I, Sohn S, Kwon HJ, Huh K, Jung MW. Estrogen blocks neurotoxic effects of beta-amyloid (1-42) and induces neurite extension on B103 cells. Neurosci Lett. 1997. 235:101–104.

24. LeBel CP, Ali SF, McKee M, Bondy SC. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol. 1990. 104:17–24.

25. Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002. 34:348–357.

26. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Dau-ssin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006. 127:1109–1122.

27. BALANCE investigators and collaborators. Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010. 375:385–395.

28. Tajes M, Gutierrez-Cuesta J, Ortuño-Sahagun D, Camins A, Pallàs M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009. 47:228–237.

29. Chong YH, Shin YJ, Lee EO, Kayed R, Glabe CG, Tenner AJ. ERK-1/2 activation mediates Abeta oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J Biol Chem. 2006. 281:20315–20325.

30. Lee EO, Park HJ, Kang JL, Kim HS, Chong YH. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J Neurochem. 2010. 112:1477–1487.

31. Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010. 285:9100–9133.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download