Abstract
Background
Systemic lymphoma can be difficult to recognize due to its diverse manifestations. Paraneoplastic leukoencephalopathy has rarely been reported in the context of lymphoma.
Case Report
We report a 45-year-old man with systemic lymphoma whose initial manifestation was sudden-onset leukoencephalopathy, mimicking stroke. This patient, who was eventually diagnosed with diffuse large B-cell lymphoma, initially presented with sudden cognitive impairment and gait disturbance. Radiological studies suggested a paraneoplastic leukoencephalopathy. Chemotherapy for lymphoma resulted in clinical and radiological improvement.
Lymphoma and leukemia are systemic diseases that affect many organs, including the central nervous system (CNS).1 These cancers may affect the CNS directly via the production of intraparenchymal or extra-axial mass lesions, or meningeal infiltration, or indirectly by coagulopathy, metabolic disturbance, or paraneoplastic antibody production.1 Atypical infections, such as John Cunningham (JC) virus or toxoplasmosis, can lead to the development of brain lesions in lymphoma patients.2 Paraneoplastic leukoencephalopathy as an initial manifestation of systemic malignancy is rare, but several reports have described its intriguing clinical course and possible pathomechanism.3,4 Although biopsy sampling is mandatory for a definitive diagnosis, it is often impossible to perform due to the location or size of the lesion, or patient refusal. The recent considerable progress in imaging technologies has provided a noninvasive modality for assessing intracranial lesions in lymphoma patients.
We present herein a case of a man with sudden gait disturbance and cognitive impairment who was initially diagnosed with cerebral infarction, but was subsequently diagnosed with paraneoplastic encephalopathy associated with systemic lymphoma.
A 48-year-old man was admitted to our hospital due to sudden gait disturbance. One month prior to the admission, he suddenly began to experience difficulty performing simple calculations and dressing himself. He had worked as a tax accountant for 20 years. This patient visited a local hospital, where he was diagnosed with cerebral infarction and treated with anticoagulation therapy; no relevant disease, medication, smoking, or alcohol history was noted. He had lost 8 kg in the 6 months prior to this event, and he was easily fatigued and experienced night sweating and general weakness. His cognitive impairment worsened over the next several weeks. He visited our hospital in order to obtain a second opinion.
On admission, the patient was alert and his orientation was intact. He was a right-handed. He was apathetic, showed right/left disorientation, and had difficulty performing simple calculations and writing. His reading and comprehension were normal. Cognitive functioning was assessed using the Mini-Mental State Examination (MMSE), on which he scored 18 points out of a possible 30; he lost 5 points on the calculation item, 3 points on memory recall, 2 points on time orientation, 1 point on writing, and 1 point on the interlocking pentagons item. The only abnormal finding on cranial nerve examination was mild dysarthria. His motor power was symmetric and preserved in all four extremities, but all muscles were moderately hypertonic. His deep-tendon reflexes were increased on both sides. He showed symmetric and intact responses to all sensory stimuli and his cortical senses were intact. His neck was supple, and carotid bruit was not audible. He exhibited small, shuffling, and hesitant steps on gait examination.
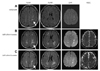
The patient's blood pressure was 107/75 mmHg, and his heart rate was 90 beats/min. We performed brain magnetic resonance imaging (MRI) and routine blood tests. Brain MRI showed multiple lesions with high signal intensity in the white matter of the bilateral hemisphere on fluid-attenuated inversion recovery and diffusion-weighted imaging (DWI), and magnetic resonance angiography showed normal intracerebral and carotid arteries (Fig. 1A). Apparent diffusion coefficient (ADC) mapping and enhanced T1-weighted imaging were not included in the MRI analyses. Blood test results showed increased liver enzymes, but serology findings were negative for hepatitis B and C viruses, the Venereal Disease Research Laboratory Test, and human immunodeficiency virus. A coagulation panel disclosed normal prothrombin and activated partial thrombin times, but the levels of C and S proteins were markedly decreased, ranging from only 30% of the normal value. He was negative for serum autoantibodies, including antinuclear antibody, lupus anticoagulant, and antineutrophil cytoplasmic antibody.
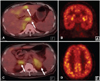
An abdominal computed tomography was performed to exclude a hepatic malignancy. This revealed multiple ill-defined, low-attenuation lesions, and multifocal, patchy, arterial, high-attenuation lesions in both lobes of the liver, with splenomegaly and bilateral adrenal gland enlargement, suggesting lymphoma. Brain MRI performed 4 weeks after the initial MRI showed enlargement of multifocal confluent subcortical lesions, with increased signal on ADC maps, suggesting an inflammatory lesion such as demyelinating disease, rather than an acute infarction (Fig. 1B). The lesions were not associated with a mass effect and showed no definite enhancement. Whole-body 18F-fluorodeoxyglucose (FDG) and positron-emission tomography (PET) revealed multiple hypermetabolic lesions in the bilateral adrenal glands, liver, and lymph nodes, as shown in Fig. 2. However, there was no alteration of brain metabolism.
A lumbar puncture study was performed to exclude CNS infection or leptomeningeal involvement of lymphoma. This showed normal opening pressure with a clear color, and two white blood cells were found, with markedly increased levels of protein (191.2 mg/dL) and glucose (92 mg/dL; serum glucose, 136 mg/dL). Polymerase chain reaction studies against viral genes, including human herpes virus types 1-6 and JC virus were all negative. There was no evidence of tuberculosis, Lyme disease, or fungal infection in staining and culture studies of a lumbar puncture specimen. Malignant cells were not found on repeated cytologic evaluation of lumbar puncture specimens. No oligoclonal band or myelin basic protein was found. The presence of paraneoplastic autoantibodies was not evaluated and a biopsy of the brain lesion was not undertaken. A liver mass biopsy disclosed diffuse large B-cell lymphoma. The patient was diagnosed with paraneoplastic leukoencephalopathy due to diffuse large B-cell lymphoma and was treated with systemic chemotherapy, including cyclophosphamide, doxorubicin, vincristine, and prednisone. His cognitive impairment and gait disturbance gradually improved over the next 2 months, he regained the ability to calculate and write letters, his MMSE score improved to 28, and he regained the ability to walk and to articulate when speaking. The final brain MRI showed that the extent of the lesion had markedly decreased (Fig. 1C).
This case signifies the diversity of CNS manifestations, which include cerebral infarction, lymphoma invasion, atypical infectious encephalitis, and paraneoplastic encephalopathy,1,2,5,6 in a patient with systemic lymphoma. The presenting sudden-onset focal neurologic deficit and high-signal-intensity lesion on DWI suggested an initial diagnosis of cerebral infarction. The combined systemic symptoms, including progressive weight loss, night sweating, and general weakness, were suggestive of systemic disease, but they were neglected. It is unlikely that a patient with cerebral infarction would have a progressive clinical course after symptom onset and slow-growing lesions on brain MRI. The newly expanded lesion had high signal values on both DWI and ADC mapping. It is therefore highly unlikely that this lesion was the result of acute infarction given that the latter exhibits a high signal value on DWI and a low ADC value during the acute stage as a result of isotropically restricted water diffusion by cytotoxic edema.7
Brain lymphoma usually presents as a single or multiple enhancing lesions in immunocompetent patients.8 However, intravascular infiltration of large B-cell lymphoma shows different MRI findings, typically involving the white matter, with minimal enhancement after contrast administration and little mass effect, and is typically referred to as intravascular lymphomatosis.5 It has been reported that the ADC values of CNS lymphomas are lower than those of other intracerebral tumors and are close to those seen in acute infarction.9 Intravascular infiltration of large B-cell lymphoma shows low ADC values due to combined ischemic brain injuries.10 However, this patient had a lesion with high signal values on ADC mapping. Additional studies were performed to exclude CNS malignancy.
FDG PET allows the evaluation of metabolic activity and provides additional information that aids in the differentiation between lymphoma and nonneoplastic conditions, including CNS lymphoma.11 The brain lesion in our patient did not show increased metabolism, suggesting that it was not neoplastic. Cytologic evaluation of repeated lumbar puncture specimens did not show malignant cells, and the protein level was markedly increased, suggesting an active inflammatory or demyelinating process. Lumbar puncture specimens were checked repeatedly for the presence of viral genes, and the results were consistently negative against JC virus and other infectious pathogens. The findings of brain MRI, FDG PET, and lumbar puncture studies strongly suggested that the brain lesion in our patient did not involve direct lymphoma invasion or infectious disease, but rather resulted from an active inflammatory disease such as demyelinating encephalopathy. Our patient showed a good response to systemic chemotherapy, suggesting paraneoplastic leukoencephalopathy as the most probable diagnosis.
There have been a few reported cases of combined demyelinating CNS lesions in patients with systemic malignancy.3,4 There has been one report of diffuse encephaloradiculopathy involving the cerebrum, brainstem, cerebellum, and peripheral nerves in a patient with silent hepatocellular carcinoma.3 Tumor-associated immunological interruption was suggested as a possible mechanism underlying diffuse demyelination of the nervous system.3 Another case report describes acute multifocal CNS demyelination as the initial presentation of lung adenocarcinoma without CNS metastasis.4 The pathomechanism may involve paraneoplastic autoantibodies or immunomodulation by the malignancy itself and/or by treatment. Brainstem or temporal lobar involvement in systemic malignancy is more commonly reported than cerebral white-matter involvement, a condition known as limbic encephalitis, which is often associated with several autoantibodies, including the anti-Hu antibody.4 However, self-limiting, acute, disseminated encephalomyelitis cannot be excluded as a possible diagnosis in this case.12
The findings of this case suggest that systemic lymphoma involves the CNS in the form of paraneoplastic encephalopathy. It must be differentiated from cerebral infarction, direct lymphoma invasion, and atypical infection in order to determine the therapeutic plan and accurately forecast the prognosis. This case also suggests that systemic malignancy must be considered in the differential diagnosis of young patients with sudden-onset neurological deficits due to brain lesions involving multifocal white matter.
Figures and Tables
Fig. 1
Brain MRI data. Brain MRI showing multifocal white-matter lesions with high signal intensity on fluid-attenuated inversion recovery (FLAIR), MRI, and DWI, with an increased apparent diffusion coefficient value. Compared to the initial image (A), the lesions were markedly increased after 4 weeks (B), and then decreased following systemic chemotherapy (C). DWI: diffusion weighted image, ADC: apparent diffusion coefficient.
References
2. Yoon JH, Bang OY, Kim HS. Progressive multifocal leukoencephalopathy in AIDS: proton MR spectroscopy patterns of asynchronous lesions confirmed by serial diffusion weighted imaging and apparent diffusion coefficient mapping. J Clin Neurol. 2007. 3:200–203.

3. Phanthumchinda K, Rungruxsirivorn S. Encephaloradiculopathy: a non-metastatic complication of hepatocellular carcinoma. J Med Assoc Thai. 1991. 74:288–291.
4. Gonzales N, Jarboe E, Kleinschmidt-DeMasters BK, Bosque P. Acute multifocal CNS demyelination as first presentation of systemic malignancy. Neurology. 2005. 65:166.

5. Song DK, Boulis NM, McKeever PE, Quint DJ. Angiotropic large cell lymphoma with imaging characteristics of CNS vasculitis. AJNR Am J Neuroradiol. 2002. 23:239–242.
6. Brecher K, Hochberg FH, Louis DN, de la Monte S, Riskind P. Case report of unusual leukoencephalopathy preceding primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1998. 65:917–920.

7. Lövblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol. 1998. 19:1061–1066.
8. Fleming JO, Keegan BM, Parisi JE. A 52-year-old man with progressive left-sided weakness and white matter disease. Neurology. 2007. 69:600–606.

9. Karaarslan E, Arslan A. Diffusion weighted MR imaging in non-infarct lesions of the brain. Eur J Radiol. 2008. 65:402–416.

10. Baehring JM, Henchcliffe C, Ledezma CJ, Fulbright R, Hochberg FH. Intravascular lymphoma: magnetic resonance imaging correlates of disease dynamics within the central nervous system. J Neurol Neurosurg Psychiatry. 2005. 76:540–544.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download