Abstract
Background and Purpose
Low-dose topiramate (TPM) monotherapy has recently been found effective for seizure control in newly diagnosed epilepsy. In higher dosages, TPM has been associated with relatively high rates of adverse cognitive effects; similar side effects have been seen after rapid titration or polytherapy. However, its cognitive effects during low-dose monotherapy have not been established. We evaluated the cognitive effects of low-dose TPM compared with oxcarbazepine (OXC), a drug that does not appear to affect cognitive function.
Methods
Cognitive tests and subjective complaints of 30 patients with low-dose TPM monotherapy (50-200 mg/day) were retrospectively compared with those of 30 patients with OXC monotherapy at 1 year of medication. The two groups did not differ with respect to epilepsy-relevant variables, nor on baseline neuropsychological tests.
Results
The TPM group showed a significant difference in the performance of delayed word recall (P<0.05), backward digit span (P<0.01), and verbal fluency (P<0.05) compared with the OXC group. The TPM group showed worse performances of digit span and verbal fluency. The OXC group showed better performances of delayed word recall. The incidence of cognitive complaints was higher in the TPM group (50%) than in the OXC group (20%) (P<0.05). These cognitive effects shown in the TPM group were dose-related. The cognitive dysfunction was trivial with patients taking 50 mg/day TPM.
A significant proportion of patients with epilepsy are at increased risk of cognitive impairment. A variety of factors contribute to their cognitive dysfunction.1 In particular, antiepileptic drugs (AEDs) can cause adverse cognitive effects.2,3 AEDs exert a dose-dependent effect on cognitive functioning, and AED polytherapy can result in even more striking adverse cognitive effects. However, the differential effects of AED monotherapy are less clear.3
Low-dose topiramate (TPM) monotherapy has recently been found to be effective for seizure control in partial or generalized epilepsy.4,5 However, TPM has been associated with a high incidence of cognitive impairment (ranging between 11% and 20%) in patients with refractory epilepsy who have received polytherapy.6,7 The major cognitive complaints are impaired attention / concentration, memory deficits, slow thinking, and word-finding difficulties.6-14 These effects relate to higher dosages, rapid titration, and polytherapy.2,3,6 When TPM monotherapy is prescribed at dosages of 25~500 mg/day, the cognitive impairments are usually mild to moderate and the incidence is less than 10%.4,5 Although it is reasonable to assume that TPM would cause fewer cognitive deficits at lower dosages, the magnitude of such effects has not been established, and further neuropsychological studies are needed.
Oxcarbazepine (OXC) is a novel AED that is chemically related to carbamazepine and is approved for initial or add-on treatment of partial seizures.15 Two European studies have evaluated its cognitive effects in adult epilepsy patients. In one of these, newly diagnosed patients received OXC or other AEDs as a monotherapy for 4 months. Compared with baseline, OXC-treated patients improved in 1 of 20 cognitive tasks and worsened in none. Patients treated with carbamazepine, valproic acid, phenobarbital, or phenytoin (PHT) monotherapy had similar results.16 The other study was a double-blind comparison of OXC with PHT monotherapy in newly diagnosed patients,17 in which no differences between OXC and PHT were detected in any of the seven cognitive variables measured at any of the time points. We have also previously investigated the cognitive effects of OXC in epilepsy patients using neuropsychological tests and event-related potentials, and did not find any negative effects of OXC on cognition after 1 year of treatment.18 The results of these studies suggest that OXC does not affect cognitive function in epilepsy patients.
The aim of this study was to identify cognitive effects of low-dose TPM compared with OXC in epilepsy patients treated with these drugs for 1 year. A variety of cognitive tests and subjective complaints were compared at baseline and 1 year of medication between two groups.
Our epilepsy clinic began to study the cognitive effects of AEDs on epilepsy patients in 2000. We routinely performed cognitive evaluations (i.e., records of subjective complaints and neuropsychological tests) after AED was taken. The severity of subjective complaints after medication was divided into four grades: Grade 0 meant no symptoms; Grade 1 meant mild and intermittent cognitive problems, usually of no consequence; Grade 2 meant moderate and steady cognitive deficits, sometimes creating discomfort; and Grade 3 equated with severe cognitive deficits such that medication had to be discontinued. The neuropsychological tests were scheduled to perform three times on each subject: at baseline, at 1 year, and after 3 years of medication. EEGs were performed on all patients before entering the study and also at the time of the second and third neuropsychological test. The EEGs included hyperventilation, photic stimulation, and sleep provocation and they were interpreted by the same epileptologist who was blinded to the clinical data. MRI of the brain was performed once on each patient during the study.
More than 600 cases were enrolled in our epilepsy neuropsychological database. From this collection of documented cases we selected subjects if they matched all of the following criteria: (1) TPM or OXC monotherapeutic patients who were undergoing follow-up cognitive evaluations at 1 year, (2) TPM dosage less than 200 mg/day, (3) either newly diagnosed epilepsy or had epilepsy that had not been treated with AEDs for more than 6 months, (4) no progressive neurological disorders, head injury, mental retardation, alcohol or drug abuse, ongoing use of any central-acting medications, severe psychiatric problems, or other severe medical disorders, and (5) no cognitive complaints at baseline.
In total, 30 patients for TPM therapy and 38 for OXC met the above criteria, with 30 of the latter patients being age-matched with the TPM group to form a comparison group. Patient characteristics are listed in Table 1. Daily TPM dosages at 1 year were 50 mg for 9, 75 mg for 10, 100 mg for 8, and 200 mg for 3 patients (mean dose: 87 mg). Daily OXC dosages at 1 year were 600 mg for 10, 750 mg for 1, 900 mg for 17, 1,200 mg for 1, and 1,500 mg for 1 patient (mean dose: 825 mg). Both groups showed a significant decrease of their monthly seizure rate and EEG abnormalities after AED medication. The groups did not differ with respect to epilepsy-relevant variables.
Cognitive tests were administered in a sound-attenuated, temperature-controlled room. All the tests were performed by a single examiner. According to the literature and our own clinical experience, we selected a few cognitive measures as being particularly sensitive to AED-induced cognitive impairment. We assessed memory function through list learning, immediate and delayed word recall, word recognition, and visual reproduction based on the Memory Assessment Scale, obtained from Psychological Assessment Resources.19 We assessed attention deficit by using digit spans (forward and backward) from the Wechsler Memory Scale-Revised.20 We examined attention, visuomotor tracking abilities, and mental flexibility by using the Trail Making Test (TMT) from the Halstead-Reitan Battery.21 We studied verbal fluency by using semantic fluency tests from the BDAE-3 (Boston Diagnostic Aphasia Examination-Third Edition).22 Testing sessions lasted about 30 minutes. In the rare case of seizures occurring during neuropsychological examination, testing was suspended and data were not evaluated.
We evaluated the differences in cognitive function, on the basis of subjective complaints and cognitive tests, between the TPM and OXC groups at 1 year. We also investigated the change in cognitive scores from baseline to 1 year of medication, in each group. We compared cognitive results with the patients' characteristics, the clinical features of the epilepsy, EEG abnormalities, neuroradiological findings, and daily AED dosage. To assess the effects of daily AED dosage on cognitive function, the frequency of cognitive complaints and the change in cognitive scores from baseline to 1 year of medication, at each target dose, were compared.
For nearly all the neuropsychological tests a higher score indicated a better performance; an exception was the TMT (parts A and B), where a lower score indicated a better performance because here the time required was defined as the dependent measure. The data for continuous variables are expressed as mean±SD values, and the data for categorized variables are expressed as frequencies. We used paired t tests to compare cognitive scores before and after medication in the same subject. We also used t tests for independent samples to compare the cognitive scores of the TPM group with those of the OXC group. Fisher's exact test was used for the categorized variables. A one-way analysis of variance (ANOVA) for independent samples was used for comparing differences of cognitive scores before and after AED medication among groups receiving different AED dosages. Bonferroni correction was employed for post-hoc comparisons, with an α level set at 0.05.
At 1 year, as shown in Table 2, the TPM group showed worse performances of forward and backward digit span (P<0.01) and verbal fluency (P<0.01) compared with baseline. The OXC group showed better performances of list learning (P<0.05), delayed word recall (P<0.05), and Trail Making Test parts A (P<0.05) and B (P<0.01). Patient groups were compared with respect to several neuropsychological tests done at baseline. As Table 2 indicates, their initial cognitive scores were not significantly different from each other. However, after 1 year of treatment the TPM group showed a significant difference in the performances of delayed word recall (P<0.05), backward digit span (P< 0.01), and verbal fluency (P<0.05) compared to the OXC group. The differences in the performance of backward digit span and verbal fluency between groups were related to the worse performance of these tests in the TPM group due to a significant decrease in those scores after 1 year. On the other hand, the difference in the delayed word recall was due to the improved performance on this test by the OXC group over the course of treatment.
The incidence of cognitive complaints was higher (P< 0.05) in the TPM group (15/30 patients, 50%) than the OXC group (6/30 patients, 20%). Patients taking TPM described their cognitive complaints as memory deficit (50%), speech problems (30%), attention / concentration deficit (10%), and psychomotor slowing (7%). The severity of symptoms was expressed as Grade 1 in 12 patients and Grade 2 in 3 patients. Patients taking OXC complained of memory deficit (17%) and attention / concentration deficit (3%), which were expressed as Grade 1.
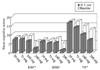
The frequency of cognitive complaints and changes in neuropsychological test scores in the TPM and OXC groups did not correlate with patient characteristics, clinical features of epilepsy, EEG abnormalities, or neuroradiologic findings. However, the frequency of cognitive complaints in the TPM group was significantly lower in patients taking 50 mg/day TPM (1/9 patients) than those taking 75 mg/day (7/10 patients), and 100 mg/day (6/8 patients) (Fisher exact, P<0.05). One-way ANOVA revealed a significant interaction effect between the amount of daily TPM dosages and the differences in cognitive score before and after TPM medication on digit span forward (P<0.05), digit span backward (P<0.05), and verbal fluency (P<0.05) tests. After post-hoc comparisons, groups taking 100 mg/day and 200 mg/day TPM showed significant decreases in digit span and verbal fluency compared to those in the group taking 50 mg/day TPM (P<0.05). As shown in Fig. 1, the group prescribed 50 mg/day TPM did not show any significant change of scores on these tests at 1 year.
This was the first comparative study between TPM and OXC demonstrating the cognitive dysfunctions of low-dose TPM (75~200 mg/day) in epileptic patients. After 1 year of treatment, the TPM group showed a significant difference in the performance on delayed recall, backward digit span, and verbal fluency compared with the OXC group. The incidence of cognitive complaints was higher in the TPM group than in the OXC group. Low-dose TPM monotherapy had negative effects on attention/concentration and verbal fluency, although it reduced monthly seizure rate and EEG abnormalities to be related to the cognitive impairment. These cognitive effects were dose-related. Cognitive dysfunction was trivial with patients taking 50 mg/day TPM. On the other hand, OXC has a positive effect on cognition such as list learning, delayed word recall, and TMT parts A and B.
Even though several studies have provided clinical evidence of TPM-induced cognitive impairment, their findings were limited by small numbers of patients, the absence of baseline cognitive assessments, intractable seizures, irregular TPM dosages, concurrent or previous AED use, or brain pathology.6-14 To minimize these confounding factors, the ideal cognitive study should involve patients with newly diagnosed epilepsy on AED monotherapy, with cognitive assessments before treatment, and at a point when the patients have been in steady-state treatment at a certain clinical dose.26,27 Our cases met these criteria and we somewhat eliminated several of the confounding factors in epilepsy. We selected patients who were taking monotherapy. A substantial portion of our cases were newly diagnosed and nonlesional. The majority of cases were well controlled by AED.
Another common methodology in studies of the cognitive effects of AEDs is a comparative one, in which the cognitive effects of the drug of interest is compared with other widely prescribed drugs. However, results of such studies can be obscured by differences in populations, seizure types, seizure frequency, duration of treatment, dosage, and background therapy (i.e., monotherapy or polytherapy).25 Our study once again overcame these problems. Nevertheless, since this study was retrospective, it did have some inevitable methodological limitations. Patients were selected from our neuropsychological database according to a set of self-generated inclusion criteria; selection bias therefore cannot be excluded, and so a future multicenter, double-blind, and placebo-controlled study would be invaluable.
As evident from the incidence of subjective complaints, TPM is better tolerated at lower dosages. In recent monotherapeutic trials of TPM, the incidence of cognitive complaints ranged from 3% to 11% when using 25~500 mg/day TPM.4,5,26 Compared to those trials, the incidence of cognitive complaints in our study was considerably higher (50%). In fact, the incidence in our study was even higher than that of a study using 400 mg/day TPM as adjunctive therapy (where memory deficit was 18%, difficulty with concentration/attention was 9%, and language disorders were 12%).10 These differences might have been due to ethnic variations because dosage requirements and the potential for toxic reactions to psychotropic medication can differ with race and ethnicity.27 Our recommendation is that Asians should have their dosage of TPM adjusted more cautiously than is the case with Caucasians. Another possible explanation for the higher incidence of cognitive complaints in our study is that TPM patients in previous trials had had poor awareness of their cognitive dysfunction and so they may not have reported these problems spontaneously.13 Therefore, specific questioning about cognitive problems and brief cognitive testing during patients' clinic visits may be particularly important when managing TPM treatment.
It remains to be elucidated whether there is any correlation between daily TPM dosages and cognitive impairment. Cognitive complaints tend to be dose-related; 5,26 however, performance on cognitive tests is not.9,13 Our study showed that both indices were certainly affected by daily TPM dosages of more than 50 mg. Data from controlled trials suggest that 100 mg/day TPM as monotherapy is effective for the treatment of epilepsy.28 However, this does not mean that all patients will respond to this dosage and experience clinical benefit; lower or higher doses may better suit individual patients. Recently, a 59% seizure-free rate at 1 year using 50 mg/day TPM has been reported in patients with newly diagnosed epilepsy.26 Among 9 patients who took 50 mg/day TPM in our study, 7 patients became seizure-free at 1 year and most of them (6 of 7 patients) had been diagnosed as newly diagnosed epilepsy. Therefore, we recommend 50 mg/day TPM as a target dosage for the treatment of newly diagnosed epilepsy in order to minimize cognitive impairment.
Findings of significant changes in digit span can be considered a reflection of impaired attention / concentration (i.e., working memory). Interestingly, our study showed that the learning and memory tests were not affected by TPM, despite a high incidence of memory complaints. This could mean that memory deficits are related to impairments in attention. Our patients mostly complained of short-term memory impairments such as holding in mind a spoken telephone number or address while they searched for a pencil and paper. Since the impairments of working memory and verbal fluency revealed by the current study reflect frontal lobe dysfunction,13 further cognitive measures for this condition, probably involving neuroimaging techniques, might be useful in clarifying the pathophysiology of cognitive dysfunction induced by TPM.
The OXC group in our study showed better performances on attention and memory tests and less cognitive complaints than did the TPM group. The positive cognitive effects of using OXC may be related to its therapeutic effect on epilepsy, which lead to decreased psychosocial problems, or to stimulation of psychomotor functioning. In comparing OXC to placebo, a double-blind, low-dose, cross-over study with healthy volunteers indicated that OXC improved performance on both a focused attention task and on manual writing speed.29 However, further studies are needed to elucidate its cognitive effect on epilepsy patients.
Avoiding the harmful cognitive effects of AEDs is especially important to those requiring maximal cognitive efficiency for their job, school, or daily activities. Even low-dose TPM has a negative effect on short-term memory and verbal fluency compared with OXC, an effect that could compromise occupational functioning or academic achievement. Awareness of this risk, and careful monitoring of patients using TPM, will help minimize long-term problems. Lower dosages such as 50 mg/day TPM could not only decrease the risk of significant cognitive effects, but also maintain its efficacy. Further studies are needed to clarify the optimal daily dosage of TPM for maximizing treatment effectiveness and improving the patient's quality of life, especially in newly diagnosed epilepsy.
Figures and Tables
Figure 1
Comparison of the difference in mean cognitive scores before and after initiation of TPM medication among groups with different daily TPM dosages (50 mg, n=9; 75 mg, n=10; 100 mg, n=8; and 200 mg, n=3). A significant interaction effect on the digit span and verbal fluency tests is observed. A group assigned to take 50 mg/day TPM did not exhibit any significant change in scores on these cognitive tests compared with other groups. DSF; digit span forward, DSB; digit span backward, VF; verbal fluency. *P<0.05 (one-way ANOVA for independent samples).
ACKNOWLEDGMENT
The authors thank Geum-Ye Bae, who is a neuropsychologist, for conducting the cognitive tests.
References
2. Aldenkamp AP, De Krom MD, Reijs R. Newer antiepileptic drugs and cognitive issues. Epilepsia. 2003. 44:Suppl 4. 21–29.

3. Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004. 5:Suppl 1. S60–S65.

4. Gilliam FG, Veloso F, Bomhof MA, Gazda SK, Biton V, Ter Bruggen JP, et al. A dose-comparison trial of topiramate as monotherapy in recently diagnosed partial epilepsy. Neurology. 2003. 60:196–201.

5. Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003. 107:165–175.

6. Bootsma HP, Coolen F, Aldenkamp AP, Arends J, Diepman I, Hulsman J, et al. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004. 5:380–387.

7. Tatum WO 4th, French JA, Faught E, Morris GL 3rd, Liporace J, Kanner A, et al. Postmarketing experience with topiramate and cognition. Epilepsia. 2001. 42:1134–1140.

8. Aldenkamp AP, Baker G, Mulder OG, Chadwick D, Cooper P, Doelman J, et al. A multicenter, randomized clinical study to evaluate the effect on cognitive function of topiramate compared with valproate as add-on therapy to carbamazepine in patients with partial-onset seizures. Epilepsia. 2000. 41:1167–1178.

9. Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000. 69:636–641.

10. Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003. 60:1483–1488.

11. Mula M, Trimble MR, Thompson P, Sander JW. Topiramate and word-finding difficulties in patients with epilepsy. Neurology. 2003. 60:1104–1107.

12. Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, et al. The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia. 2003. 44:339–347.

13. Kockelmann E, Elger CE, Helmstaedter C. Significant improvement in frontal lobe associated neuropsychological functions after withdrawal of topiramate in epilepsy patients. Epilepsy Res. 2003. 54:171–178.

14. Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005. 6:373–381.

15. Beydoun A, Sachdeo RC, Rosenfeld WE, Krauss GL, Sessler N, Mesenbrink P, et al. Oxcarbazepine monotherapy for partial-onset seizures: a multicenter, double-blind, clinical trial. Neurology. 2000. 54:2245–2251.

16. Saber A, Møller A, Dam M, Smed A, Arlien-Soborg P, Buchman J, et al. Cognitive function and anticonvulsant therapy: effect of monotherapy in epilepsy. Acta Neurol Scand. 1995. 92:19–27.

17. Aikia M, Kalviainen R, Sivenius J, Halonen T, Riekkinen PJ. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow-up. Epilepsy Res. 1992. 11:199–203.

18. Park SP, Hwang YH, Kim JI, Kim JY, Kwon SH, Jung BW, et al. Cognitive function in epileptic patients treated with oxcarbazepine: neuropsychologic test and event-related potential. J Korean Neurol Assoc. 2002. 20:27–33.
19. Williams JM. Memory Assessment Scales Professional Manual. 1991. Odessa, FL: Psychological Assessment Resources.
20. Wechsler D. Wechsler Memory Scale-Revised Manual. 1987. San Antonio, TX: The Psychological Corporation.
21. Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 1993. 2nd edn. Tucson, AZ: Neuropsychology Press.
22. Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination-Third Edition (BDAE-3). 2000. San Antonio, TX: Psychological Corporation.
23. Aldenkamp AP. Cognitive and behavioural assessment in clinical trials: when should they be done? Epilepsy Res. 2001. 45:155–157.

24. Thompson P. Cognitive and behavioural assessment in clinical trials: when should they be done? Epilepsy Res. 2001. 45:159–161.

25. Bourgeois BFD. Determining the effects of antiepileptic drugs on cognitive function in pediatric patients with epilepsy. J Child Neurol. 2004. 19:Suppl 1. S15–S24.

26. Arroyo S, Dodson WE, Privitera MD, Glauser TA, Naritoku DK, Dlugo DJ, et al. Randomized dose-controlled study of topiramate as first-line therapy in epilepsy. Acta Neurol Scand. 2005. 112:214–222.

27. Lin KM, Poland RE, Lesser IM. Ethnicity and psychopharmacology. Cult Med Psychiatry. 1986. 10:151–165.

28. Silberstein SD, Ben-Menachem E, Shank RP, Wiegand F. Topiramate monotherapy in epilepsy and migraine prevention. Clin Ther. 2005. 27:154–165.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download