Abstract
Human infection by Rhodococcus species is rare and mostly limited to immunocompromised hosts such as patients infected with the human immunodeficiency virus (HIV) or organ transplant recipients. The most common strain is R. equi, and the most common clinical presentation is pulmonary infection, reported in 80% of Rhodococcus spp. infections. The central nervous system is an uncommon infection site. We report a case of a patient with pneumonia, brain abscess, and recurrent meningitis caused by Rhodococcus spp. He initially presented with pneumonia with necrosis, which progressed to brain abscess and recurrent meningitis. Rhodococcus spp. was identified from the cerobrospinal fluid (CSF) collected during his fourth hospital admission. Despite prolonged treatment with appropriate antibiotics, meningitis recurred three times. Finally, in order to administer antibiotics directly into the CSF and bypass the blood-brain barrier, an Ommaya reservoir was inserted for administration of 90 days of intrathecal vancomycin and amikacin in conjunction with intravenous and oral antibiotics; the patient was finally cured with this treatment regimen.
Rhodococcus spp. was first isolated in 1923 [1], and the first human infection was reported in 1967 [2]. Human infection is rare and mostly limited to immunocompromised hosts such as patients with human immunodeficiency virus (HIV) or organ transplant recipients. [134567]. The most common strain is R. equi, and the most common infection site are the lungs, with pneumonia, nodule, or abscess with or without pleural involvement. A previous report on 107 R. equi isolates (101 human and six animal isolates) obtained positive samples from blood (32 isolates), sputum (30), lung tissue (13), and other sites (32); only three of the 107 isolates were obtained from the cerebrospinal fluid (CSF) [8]. R. equi infections can occur at both single and multiple sites and may also develop additional sites of disease during antibiotic therapy. Relapses are common at the initial site of infection and at distant locations [16].
We experienced a case of pneumonia, brain abscess, and recurrent meningitis due to Rhodococcus spp. infection, which was successfully treated by intrathecal injection of antibiotics through an Ommaya reservoir. To our knowledge, this is the first reported case of brain abscess and meningitis caused by Rhodococcus spp. in South Korea.
A 48-year-old man visited the emergency room (ER) because of a high fever (39℃), yellowish sputum, and a cough that had persisted for seven days. Three days prior, right chest wall pain had developed. He had been diagnosed with systemic lupus erythematosus (SLE) and idiopathic thrombocytopenic purpura (ITP) seven years previously, and had been taking 100 mg cyclosporine twice daily and 25 mg prednisolone daily. He had also been diagnosed with diabetes mellitus six years prior and had been taking sitagliptin and metformin. He had been working at a fertilizer plant, but had retired six months before. The physical examination revealed an alert mental state. His blood pressure was 110/75 mmHg, body temperature was 38.4℃, heart rate was 73 beats/min, and respiratory rate was 20 breaths/min. His heart sounds were normal, but coarse crackling was detected in both lower lungs, especially the right lower lung field. There was no specific finding in his abdomen, back, or extremities. His white blood cell count (WBC) was 13,800/mm3 (91.9% neutrophils and 8.1% monocytes), hemoglobin level was 10.1g/dL, and platelet count was 269,000/mm3. His C-reactive protein concentration was 23.9 mg/dL (reference range: 0.00-0.30 mg/dL), and total bilirubin, aspartate aminotransferase, and alanine aminotransferase concentrations were 1.15 mg/dL (0.20-1.20 mg/dL), 12U/L (5-40U/L), and 13 U/L (5-45U/L), respectively. Chest radiography revealed mass-like consolidations with necrosis in the left and right lower lung basal territory. A chest computed tomography (CT) with enhancement revealed findings consistent with the suspected necrotizing bacterial pneumonia (Fig. 1A). Moxifloxacin and clindamycin were administered intravenously for 10 days, but neutropenia, possibly due to moxifloxacin, occurred, and the antibiotics were changed to 2 g ceftriaxone per day and 150 mg roxithromycin twice daily for another 14 days. Despite prolonged antibiotic therapy, his high fever and pneumonic infiltrations persisted; however, no microorganisms were isolated from sputum or blood cultures. On the 26th hospital day (HD), bronchoalvelolar lavage was performed, but no pathogens could be identified. After switching back to moxifloxacin and clindamycin, the patient's symptoms alleviated and signs improved (Fig. 1B). On the 49th HD, the patient developed headache and diplopia with fever and was transferred to the infectious disease department. Brain magnetic resonance imaging (MRI) with enhancement showed multiple aggregated enhancing lesions in his left cerebellum, midbrain, pons, and supratentorial parenchyma with surrounding edema (Fig. 2A). Suspecting sepsis with hematogenous brain abscess, 2 g/day vancomycin and 4 g/day ceftriaxone were started and continued for six weeks. Blood cultures performed several times after the episode were negative for growth, and transesophageal echocardiography to examine infective endocarditis did not show vegetation. After six weeks of treatment, the patient improved and was discharged without neurological symptoms or signs. The medical history of the patient, including the six hospitalization events, is illustrated in Figure 3.
Seven days after discharge, the patient revisited the ER with recurrent fever, headache, and diplopia (second admission). His blood pressure was 100/60 mmHg, his body temperature was 38.0℃, his heart rate was 90 beats/min, and his respiratory rate was 20 breaths/min. His mentality was clear and no other neurological signs were detected except diplopia. A chest CT taken in the ER revealed that previous lung involvement was improved (Fig. 1C). An MRI taken in the ER revealed an improved brain abscess (Fig. 2B). Suspecting insufficient treatment for the brain abscess, vancomycin and ceftriaxone were administered for 25 days and he was discharged with antibiotics, switching to teicoplanin 400 mg every 24 hours during outpatient treatment for 23 days (Fig. 2C).
On the 25th day after the previous hospitalization (teicoplanin treatment during outpatient care) he was again admitted through the ER (third admission). The patient had a fever, headache, nausea, and vomiting. Enhancement of meninges at the tegmentum, right corpus splenium, and left corona radiate were seen on contrast-enhanced CT scans. A CSF study suggested bacterial meningitis, with a leukocyte count of 640/mm3 (87% polymorphocytes and 13% monocytes), protein concentration of 186 mg/dL, and glucose level of 79 mg/dL. Several CSF and blood cultures were negative, and adenosine deaminase values of initial and subsequent CSF studies were 9.9 and 5.9 U/L (reference range 0-8.0 U/L), respectively. Ceftriaxone and vancomycin were re-administered with improvement of the symptoms and signs. On the 36th day of ceftriaxone and vancomycin treatment, his fever rose to 39℃, and a CSF study revealed 880 leukocytes/mm3 (94% polymorphocytes and 6% monocytes), a protein concentration of 122 mg/dL, and a glucose level of 78 mg/dL. Suspecting development of ceftriaxone and vancomycin resistance, the treatment regimen was changed to meropenem and linezolid (6 g/day and 1,200 mg/day, respectively) for two weeks. The fever subsided and the patient was discharged.
Five days after his discharge, the patients again visited the ER complaining of fever, headache, and nuchal rigidity (fourth admission). A CSF study, blood cultures, and CSF cultures were repeated. For the first time, a pathogen was isolated from CSF culture, which was identified as Rhodococcus spp.. Under a diagnosis of necrotizing pneumonia, brain abscess, and recurrent meningitis caused by Rhodococcus spp. due to insufficient treatment duration, meropenem and linezolid were administered for three weeks, the parenteral regimen was switched to 750 and 600 mg/day oral levofloxacin and rifampin, respectively, and the patient was discharged.
He was hospitalized again seven days after discharge (fifth admission). Insufficient penetration of levofloxacin into the CSF was suspected as cause of his recurrent meningitis. The meningitis was treated with 3 g/day imipenem and (5 mg/kg/day trimethoprim) trimethoprim-sulfamethoxazole (SMX) to increase CSF concentration of the antibiotics. However, one week after starting the imipenem and SMX treatment, patient had not improved; the regimen was therefore changed to linezolid (1,200 mg/day), amikacin (15 mg/kg/day), SMX, and doxycycline (400 mg/day). The patient improved after 14 days of this treatment, and he continued this regimen for an additional two months with 1,200 mg/day oral linezolid, doxycycline and SMX (SMX was switched back to meropenem because of SMX side effects after one month).
The patient again visited the ER 10 days after his previous discharge, presenting with fever, nausea, and vomiting (sixth admission). A CSF study performed that day was normal, but his WBC count was 600/mm3. He complained of a severe tingling sensation and paresthesia on both lower legs. Suspecting linezolid toxicity, which had been administered for a total of 90 days, all antibiotics were discontinued. Seven days after discontinuing antibiotics, his fever relapsed. A CSF study showed another relapse of his meningitis, with WBC 68/mm3 (95% neutrophils and 5% lymphocytes), protein 56 mg/dL, and glucose 103 mg/dL (Fig. 3). Amikacin, imipenem, doxycycline, and rifampin were administered, resulting in improvement of his symptoms. However, his fever relapsed after three weeks of this antibiotic regimen. A subsequent CSF study showed that the meningitis had worsened: his WBC was 370/mm3 (95% neutrophils and 5% lymphocytes), protein concentration was 80 mg/dL, and his glucose level was 96 mg/dL (Fig. 3). At this point, because no other therapeutic option was available, we inserted an Ommaya reservoir in order to introduce antibiotics directly into CSF, bypassing the blood brain barrier (BBB) because it was suspected that BBB might be a main cause of meningitis treatment failure, despite the risk of Ommaya reservoir infection by Rhodococcus spp.. After insertion of the reservoir, 25 mg each vancomycin and amikacin were injected intrathecally via the Ommaya reservoir, along with intravenous vancomycin (2 g/day). In addition to the intravenous and intrathecal vancomycin, doxycyline (100 mg twice daily) and rifampin (600 mg) were administered orally for two months (intravenous vancomycin was administered for 1 month and rifampin was stopped at 1.5 months because of side effects). After two months of treatment, azithromycin was administered instead of intrathecal aminoglycoside because the patient developed hearing difficulty. The total duration of intrathecal injection of vancomycin and oral doxycycline was three months. Since then, the meningitis has not recurred for four months with close follow-up. We therefore consider his Rhodococcus spp. meningitis to be in complete remission.
R. equi is the major Rhodococcus spp. strain associated with human infection. There are no standardized treatment regimens for Rhodococcus spp. infection and no standards for determining antibiotic susceptibility. In this case, Rhodococcus spp. was cultured only once during the patient's medical history, which consisted of six hospital admissions. However, several factors contributed to our final diagnosis and successful treatment of pneumonia, brain abscess, and recurrent meningitis caused by Rhodococcus spp. Firstly, the patient showed signs typical of Rhodococcus spp. infections: it occurs mostly in immunosuppressed patients [134567], pulmonary infection is the most common clinical presentation [18], and infections are known to recur commonly at primary or distant metastatic sites [1456]. The patient had been treated with immunosuppressant agents for SLE and ITP, and had initially presented with pneumonia. Secondly, fertilizer or foals are main source of these bacteria [1]; he had previously worked in a fertilizer plant, surrounded by livestock manure. A potential route of infection may have been inhalation of the microorganisms while making fertilizers. Thirdly, the case showed typical recurrent infection episodes; it began as necrotizing pneumonia, and progressed into brain abscess and three instances of recurrent meningitis. Finally, there are several possibilities to explain the failure to identify the pathogen during his initial and subsequent admissions: in his first admission, the sputum culture results may have revealed normal flora because it is hard to distinguish Rhodococcus spp. by Gram stain if they do not show dominant growth. Furthermore, the persistent exposure to antibiotics in this case affected the ability to successfully culture the microorganism, because the infection recurred during the treatment period. Even at the fourth admission, when Rhodococcus spp. grew in the CSF, the patient was receiving oral rifampin and levofloxacin. Besides the difficulty in identification, there is no standardized method for determining antibiotic susceptibility. In several in vitro susceptibility tests done by the microbroth dilution method, R. equi appears to be susceptible to glycopeptides, macrolides, fluoroquinolones, rifampin, carbapenem, aminoglycosides, and linezolid [1891011]: briefly, no resistance to amoxicillin-clavulanate, ampicillin-sulbactam, gentamicin, and imipenem; less than 5% resistance to erythromycin, rifampin, tetracycline, and SMX; and less than 20% resistance to ciprofloxacin and norfloxacin have been observed [8].
Likewise, there are no standardized treatment regimens for Rhodococcus spp. infection. For R. equi infections, immunocompromised hosts should be treated with two or three drugs for two to six months, with two to three-week intravenous (IV) period [1]. Surgery may be a useful adjunct in certain cases. Development of resistance during treatment has been reported with β-lactam, doxycycline, rifampin, and SMX [12131415]. Furthermore, because this pathogen is an intracellular organism, several studies have recommended use of at least one antibiotic with intracellular penetration, such as erythromycin or rifampin [810]. In vitro synergy studies have shown four combinations of drugs to be effective against R. equi: rifampin and erythromycin, rifampin and minocycline, erythromycin and minocycline, and imipenem and amikacin [10]. Others have argued that bactericidal activity is more important, especially during the initial treatment phase, when both extracellular and intracellular organisms are numerous [9]. During the initial phase, antibiotic regimens based on parenteral vancomycin, and could be switched to oral agents with rifampin-erythromycin or rifampin-minocycline [9].
There are several possible explanations for the recurrent meningitis in our patient. Inappropriate antibiotic administration during treatment for pneumonia might have contributed to the initial brain abscess development, and the first meningitis relapse may have occurred due to the short-term treatment lacking an intracellular bactericidal effect, since the pathogen was not identified until the fourth admission. The blood-brain barrier is another important consideration. Nau et al. [16] has reviewed CSF penetration of antibiotics. In this case, the antibiotics administered to the patient (vancomycin, ceftriaxone, meropenem, linezolid, and amikacin) have a low area under the curve (AUC) CSF/AUC serum ratio. When meningitis is improving, the ability of these antibiotics to penetrate the CSF is reduced. Moreover, because Rhodococcus spp. is an intracellular organism, cellular transportation of antibiotics must also be considered [16]. For these reasons, the standard doses used in this case might be not have been able to reach therapeutic levels in the CSF. Rhodococcus spp. easily develops antibiotic resistance, and should therefore be treated with multiple antibiotics. Considering the clinical course of the patient, the organism might have developed resistance to several antibiotics administered during the recurrent episodes.
To overcome these treatment difficulties, an Ommaya reservoir was inserted for intrathecal administration of vancomycin and amikacin. Injection of antibiotics through the Ommaya reservoir offers both advantages and disadvantages. The major advantage is that antibiotics reliably reach therapeutic level in the CSF, a technique that was successful in our patient. The disadvantages include a possible risk of irreversible neurological deficits caused by Ommaya reservoir insertion and foreign body infection. Nevertheless, the patient was successfully cured of recurrent meningitis only with this method, and did not develop side effects, although he developed hearing difficulty due to intrathecal aminoglycoside injections.
Figures and Tables
Figure 1
Chest computed tomography scan with enhancement. (A) At first admission, lobulating contour mass-like consolidations with necrosis are visible in the left lower lobe and right lower lobe basal territory (☆). (B) Pneumonic infiltration of the left lower lobe superior segment improved, but the extent of the pneumonic consolidations increased with the newly developed internal necrotic component at the right lower lobe (arrow). (C) Pneumonic consolidations at the right lower lobe and left lower lobe superior segment showed improvement.
*(Number) is the time elapsed from the first admission date (days).
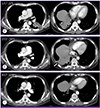
Figure 2
Brain magnetic resonance images (MRI). (A) Multiple aggregated enhancing lesions with surrounding edema in the left cerebellum, midbrain, pons, and supratentorial parenchyma (arrowheads). (B) Decreased number of enhanced lesions compared with previous MRI ( ). (C) Improvement of the previous lesions except for mild worsening of pontine lesions (☆).
). (C) Improvement of the previous lesions except for mild worsening of pontine lesions (☆).
*(Number) is the time elapsed from the first admission date (days).
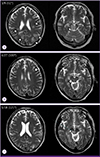
Figure 3
Clinical course of patient with necrotizing pneumonia, brain abscess, and relapsing meningitis due to Rhodococcus spp. Bold line: white blood cell counts in cerebrospinal fluid (CSF). Dotted line: body temperatures (℃).
aDay: time elapsed from the first admission date.
bCSF study: white blood cell (WBC) (/mm3), neutrophil (%), lymphocyte (%), protein (mg/dL), glucose (mg/dL).
cDuration (days) and antibiotics administered: VAN, vancomycin; TEC, teicoplanin; LZD, linezolid; CRO, ceftriaxone; MEM, meropenem; IPM-C, imipenem-cilastatin; DOX, doxycycline; AMK, amikacin; RIF, rifampin; MXF, moxifloxacin; LVX, levofloxacin; AZM, azithromycin; CLI, clindamycin; TMP/SMX, trimethoprim-sulfamethoxazole, IT, intrathecal.

References
1. Weinstock DM, Brown AE. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002; 34:1379–1385.
2. Golub B, Falk G, Spink WW. Lung abscess due to Corynebacterium equi: report of first human infection. Ann Intern Med. 1967; 66:1174–1177.
3. Lasky JA, Pulkingham N, Powers MA, Durack DT. Rhodococcus equi causing human infections: review of 29 cases. South Med J. 1991; 84:1217–1220.
4. Verville TD, Huycke MM, Greenfield RA, Fine DP, Kuhls TL, Slater LN. Rhodococcus equi infections of humans 12 cases and a review of the literature. Medicine (Baltimore). 1994; 73:119–132.
5. Weingarten JS, Huang DY, Jackman JD Jr. Rhodococcus equi pneumonia: an unusual early manifestation of the acquired immunodeficiency syndrome (AIDS). Chest. 1988; 94:195–196.
6. Kedlaya I, Ing MB, Wong SS. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin Infect Dis. 2001; 32:E39–E46.
7. Harvey RL, Sunstrum JC. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991; 13:139–145.

8. McNeil MM, Brown JM. Distribution and antimicrobial susceptibility of Rhodococcus equi from clinical specimens. Eur J Epidemiol. 1992; 8:437–443.

9. Rouquet RM, Clove D, Massip P, Moatti N, Leophonte P. Imipenem/vancomycin for Rhodococcus equi pulmonary infection in an HIV-positive patient. Lancet. 1991; 337:375.

10. Nordmann P, Ronco E. In-vitro antimicrobial susceptibility of Rhodococcus equi. J Antimicrob Chemother. 1992; 29:383–393.
11. Bowersock TL, Salmon SA, Portis ES, Prescott JF, Robison DA, Ford CW, Watts JL. MICs of oxazolidinones for Rhodococcus equi strains isolated from humans and animals. Antimicrob Agents Chemother. 2000; 44:1367–1369.

12. Samies JH, Hathaway BN, Echols RM, Veazey JM Jr, Pilon VA. Lung abscess due to Corynebacterium equi. Report of the first case in a patient with acquired immune deficiency syndrome. Am J Med. 1986; 80:685–688.

13. Nordmann P, Chavanet P, Caillon J, Duez JM, Portier H. Recurrent pneumonia due to rifampicin-resistant Rhodococcus equi in a patient infected with HIV. J Infect. 1992; 24:104–107.

14. Van Etta LL, Filice GA, Ferguson RM, Gerding DN. Corynebacterium equi: a review of 12 cases of human infection. Rev Infect Dis. 1983; 5:1012–1018.
15. Drancourt M, Bonnet E, Gallais H, Peloux Y, Raoult D. Rhodococcus equi infection in patients with AIDS. J Infect. 1992; 24:123–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download