Abstract
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia. However, it can also asymptomatically colonize the upper respiratory tract. Because of the need to distinguish between S. pneumoniae that is simply colonizing the upper respiratory tract and S. pneumoniae that is causing pneumonia, accurate diagnosis of pneumococcal pneumonia is a challenging issue that still needs to be solved. Sputum Gram stains and culture are the first diagnostic step for identifying pneumococcal pneumonia and provide information on antibiotic susceptibility. However, these conventional methods are relatively slow and insensitive and show limited specificity. In the past decade, new diagnostic tools have been developed, particularly antigen (teichoic acid and capsular polysaccharides) and nucleic acid (ply, lytA, and Spn9802) detection assays. Use of the pneumococcal antigen detection methods along with biomarkers (C-reactive protein and procalcitonin) may enhance the specificity of diagnosis for pneumococcal pneumonia. This article provides an overview of current methods of diagnosing pneumococcal pneumonia and discusses new and future test methods that may provide the way forward for improving its diagnosis.
Streptococcus pneumoniae is responsible for a spectrum of diseases, including mild but common diseases such as otitis media, sinusitis, and non-bacteremic pneumonia and serious invasive pneumococcal diseases (IPD) such as bacteremia and meningitis. Among these diseases, pneumonia demands special attention because the incidence and mortality rates of community-acquired pneumonia (CAP) are high among the elderly. In the U.S., the annual incidence rate of CAP is estimated to be 5.2 to 6.1 cases per 1000 adults, and the mortality rate may reach 2-3% [1, 2]. The mortality rate for pneumococcal CAP is higher than for general CAP: < 2-5% in adults treated as outpatients, 12% of hospitalized patients, and ≥ 25% in elderly patients with bacteremia [3, 4]. Similarly, among Korean adults, the mortality rate for general CAP is estimated to be 3.2%, while the mortality rate for pneumococcal CAP is 5.9% [5]. Since S. pneumoniae is commonly responsible for the medically serious CAP [6], it is frequently simply referred to as "pneumococcus."
Reflecting its status as a major human pathogen, S. pneumoniae was also one of the earliest pathogens to be discovered, and its microbiologic properties have been extensively investigated [7]. Pneumococci are Gram-positive bacteria with thick cell walls that contain teichoic acid (C-polysaccharide). They are capable of producing toxins (e.g., pneumolysin) as well as many surface antigens such as pneumococcal surface adhesin A (PsaA), pneumococcal choline binding protein A (PcpA), pneumococcal surface protein A (PspA), pneumococcal surface protein C (PspC), and pneumococcal autolysin A (LytA). LytA is the major autolysin responsible for lysis of pneumococci observed for mature pneumococcal cultures. However, the most prominent surface structure is the polysaccharide capsule, which is present on almost all virulent pneumococci. Pneumococci can express one of many (90+) polysaccharide capsule types that are serologically and biochemically distinct [8, 9]. Colony morphologies of two serotypes (3 and 37) are highly mucoid (Fig. 1) and distinct from other serotypes. As antibodies to the pneumococcal capsule are protective, the polysaccharide capsule is used in current vaccines. More recently, its genome sequences have been determined. Although no single gene that is unique and common to all isolates of S. pneumoniae has been reported, the genome sequences have been used to investigate pneumococcal evolution [10].
Despite its reputation as a pathogen, pneumococcus is a commensal that is often asymptomatically carried in the nasopharynxes of children and adults. Pneumococcal carriage occurs early in life, usually with a prevalence of about 30-60% in infants [11]; however, in some populations, > 90% of children are known to carry pneumococci [12]. The carriage rate may stay above 30-40% among children younger than 10 years of age, but it declines progressively until the rate reaches 1-10% among adults [11]. Since pneumococci are naturally present in the oro-nasopharyngeal space, the presence of pneumococcus in respiratory specimens does not necessarily indicate the presence of disease. Consequently, this commensalism must be incorporated in any diagnostic approaches to identifying pneumococcal infections.
In addition to S. pneumoniae, the oro- and nasopharynxes harbor Gram-negative rods and Staphylococcus aureus as well as many streptococcal species that resemble S. pneumoniae. The streptococcal species include S. mitis, S. oralis, and S. pseudopneumoniae, and are often referred as viridans species because they can produce α-hemolysis on blood agar plates similar to that produced by S. pneumoniae [13]. S. mitis and S. oralis can be responsible for subacute endocarditis and sepsis [14]. S. pseudopneumoniae is known to cause pneumonia or acute exacerbation in patients having a history of chronic obstructive pulmonary disease [15]. Gram-negative rods and staphylococci are mostly gentamicin-sensitive whereas viridans species and pneumococci are generally gentamicin-resistant. Consequently, the use of blood agar plates containing gentamicin improved the isolation of pneumococci and viridans species from respiratory specimens [16-19]. Although S. pneumoniae and viridans group are genetically related, S. mitis and S. oralis are generally resistant to optochin and bile-insoluble while S. pneumoniae is not (Fig. 2) [13]. S. pseudopneumoniae is generally bile-insoluble but is optochin-resistant only in 5% CO2, but not in room air [15, 20].
In most clinical laboratories, S. pneumoniae is routinely identified by microscopic morphology (Gram-positive bacteria in the shape of slightly pointed cocci, usually in pairs), colony morphology, and characteristic phenotypes such as α-hemolysis observed on blood agar, catalase negativity, optochin susceptibility, and bile solubility [13]. These tests exclude most [13] but not all of the viridans species [21]. Conversely, some S. pneumoniae strains may be bile-insoluble [22]. Also, viridan species may have lytA and pneumolysin genes, thereby limiting the usefulness of the genetic tests for S. pneumoniae [23]. Biochemical tests based on teichoic acid may also fail to distinguish S. pneumoniae because some viridans species can produce teichoic acid that is identical to pneumococcal teichoic acid. More recently, multi-locus sequence typing (MLST) has been adapted to differentiate among these streptococcus species [24-26]. This approach is called multilocus sequence analysis (MLSA) and is more reliable than previous methods in distinguishing among the streptococcal species but has been used only as a research method.
Thus, routine phenotyping methods used in clinical laboratories may be inadequate to definitely distinguish S. pneumoniae from viridans species. This inadequacy was illustrated by recent studies that showed that many "pneumococci" carried in the nasopharynxes of HIV patients are actually viridans species that were previously misidentified as S. pneumoniae [24]. Also, the presence of S. pneumoniae in the respiratory tract may not be pathologic in a substantial number of cases. Thus, one must be aware of these fundamental limitations when interpreting epidemiologic as well as clinical studies. This article will review conventional techniques for diagnosing pneumococcal pneumonia and recent developments in other techniques such as urinary antigen, polymerase chain reaction, and serologic tests.
Early accurate diagnosis and treatment of pneumonia are associated with improved survival; they also reduce costs associated with unnecessary investigations and complications due to inappropriate treatment [27, 28]. In a study of 14,000 elderly patients with pneumonia treated at over 3,500 hospitals, patients who received early antibiotic therapy (within 8 hours of hospital arrival) had a lower 30-day mortality [27]. In addition, etiologic diagnosis of CAP has an important effect on our ability to provide the optimal therapy for this disease, understand its societal burden, and assess the effectiveness of pneumococcal vaccines.
The diagnosis of pneumococcal pneumonia begins with establishing the presence of pneumonia. Pneumonia is often diagnosed by clinical symptoms and radiologic evidence. The clinical symptoms include cough or difficulty breathing plus tachypnea [29]. Although there could be limitations in these approaches, this review is focused on pneumococcal pneumonia, and readers are referred to other recent reviews for the limitations of pneumonia diagnosis [30-33].
S. pneumoniae is thought to be the most common etiologic agent of bacterial CAP. The gold standard in establishing pneumococcal CAP is to isolate bacteria from a normally sterile body fluid and then identify that bacterium as S. pneumoniae. To identify pneumococcal CAP from body tissue, the ideal tissue is the lung tissue obtained by biopsy or bronchoscopy; consequently, lung biopsy was used previously in research settings [34]. In addition, transtracheal aspiration has been shown to have high yields of S. pneumoniae [35, 36]. However, since these approaches are not practical in clinical settings, most bacteriological confirmation is performed with readily available fluid samples such as peripheral sputum, blood samples, and pleural fluids. Advantages and limitations of using these types of samples are described below.
Sputum Gram stains and culture are most often the first diagnostic step for pneumonia. Gram stains of sputum could be strongly suggestive of pneumococcal pneumonia if the sputum is of high-quality (< 10 squamous epithelial cells and > 25 polymorphonuclear cells at a magnification of 100×) and shows the predominant presence of Gram-positive diplococci [37, 38]. A meta-analysis of sputum studies performed in 1966-1993 found diverse sensitivity (15-100%) and specificity (11-100%) for this diagnostic method [39]. In comparison, prospective studies with high-quality sputum samples showed relatively high sensitivity (57-82%) and specificity (93-97%) [37, 40-42]. However, direct microscopic examination of Gram-stained specimens has some limitations in clinical practice; specifically, inadequate sputum collection and antimicrobial therapy before obtaining sputum specimens lead to low diagnostic yields. In the study by Musher et al. [37], sensitivity increased in inverse proportion to the duration of prior antibiotic therapy. In primary care settings, the availability of skilled microbiologists is another limitation of sputum Gram stain [39, 43]. Additional problem with this diagnostic method is that it may not be easy to collect good quality sputum from children.
Sputum culture can further assist etiology identification. However, the diagnostic sensitivity of the culture has been reported to be quite variable, ranging between 29 and 94% [36, 44-52]. Such variable results are related to inadequate sampling of sputum, delayed processing of sputum specimens, and prior antimicrobial therapy. Sputum culture was found to be negative in about 50% of patients with concurrent pneumococcal bacteremia [35, 36]. Also, Musher et al. [37] found that the sputum culture identified pneumococci only in 44% of the persons with bacteremic pneumococcal pneumonia with the sensitivity of sputum culture increasing to 93% if the comparison is confined to adequate sputum samples obtained before antimicrobial therapy. In addition, false positives may occur due to nasopharyngeal carriage, particularly among children, so the culture results should be interpreted along with the findings from Gram stain.
Since blood and pleural effusions are normally sterile, isolating pneumococci from these normally sterile tissues provides the definite diagnosis of pneumococcal pneumonia. However, the clinical utility of this diagnostic method is limited because blood cultures are estimated to be positive for S. pneumoniae in less than 10% of patients who actually have pneumococcal pneumonia [53-56]. The low rate of culture may be due to pneumonia without bacteremia, autolysis of S. pneumoniae during the stationary growth phase, use of antibiotics before the culture, or inadequate samples (e.g., insufficient blood volume) [57]. To reduce autolysis, one may have to optimize the blood culture condition and/or use an antigen-detection method or a nucleic acid amplification test (NAAT) to analyze the culture-negative broth samples from patients with a high suspicion for pneumococcal pneumonia. Nevertheless, positive blood culture unambiguously establishes pneumococcal etiology.
Empyema was once considered rare in children, but it has been increasing worldwide over the last decade [58-61]. The incidence of empyema is also increasing in adults, S. pneumoniae is likely to cause empyema in healthy young adults, and the Streptococcus milleri group is the common pathogen causing empyema in the elderly with comorbidities [62]. Conventional bacterial culture of pleural fluid is often negative among children with pneumococcal pneumonia complicated by empyema. Similar to what happens in blood cultures, the culture rate of pleural fluid may be low due to autolysin release from pneumococci during the stationary growth phase, resulting in cell death [57].
The conventional microbiological methods of pneumococcal pneumonia detection described above have several limitations. First, the culture is often falsely negative. Second, it takes several days to culture pneumococci. To overcome these limitations, laboratory methods are designed to detect pneumococcal molecules present in diverse tissue samples such as sputum, urine, breath, pleural fluids, and peripheral blood [63-66]. These methods have the theoretical advantage of rapidly detecting pneumococci even if they are non-viable after antibiotic treatment. In addition, using a novel molecular target such as PcpA may be highly desirable because its presence can clearly distinguish pneumonia from carriage: its expression requires low manganese levels, with those levels being high in the nasopharynx but low in blood [67, 68]. Nevertheless, below we describe clinical experiences with teichoic acid and capsular polysaccharide detection as they have been central to most previous investigations.
Holmberg et al. [63] compared the sensitivity and specificity of using ELISA to detect teichoic acid with the sensitivity and specificity of using latex agglutination tests to detect capsular polysaccharide antigens in sputum specimens. Both ELISA and latex agglutination tests showed favorable sensitivity (95 and 86%, respectively) and specificity (94% for both detection methods) when sputum culture was used as the standard for comparison. Recently, detection of teichoic acid has become very popular following the introduction of a rapid immunochromatographic test (Binax NOW S. pneumoniae assay) in 2003 (US Food and Drug Administration approval). This is a point-of-care test with high analytical sensitivity for C-polysaccharide in urine specimens.
Studies of urine samples from children showed inadequate specificity for the Binax NOW assay (50-60%) due to the high rate of nasopharyngeal carriage in children, resulting in urinary excretion of teichoic acid [69-73]. When concentrated children urine was used (25-fold concentration by ultra-filtration), the specificity of the Binax NOW assay decreased even more, to about 12% [70]. Because false-positive tests are common, the Binax NOW S. pneumoniae assay is not recommended for the diagnosis of pneumococcal pneumonia in children [33].
Although the specificity of the Binax NOW assay was disappointing for urine samples from children, its sensitivity (71-96%) and specificity (71-100%) for pneumococcal empyema were high when pleural fluid samples were examined (Table 1) [74-79]. Also, Gram stain of pleural fluid is rather insensitive for pneumococcal empyema, although it has a good positive predictive value [80]. Thus, the Binax NOW S. pneumoniae assay may be practically useful for the diagnosis of pneumococcal empyema using pleural fluid samples. The study by Le Monnier et al. [80] also found that NAAT could improve the detection of etiologic agents in 43% of patients with pneumococcal empyema.
Clinical studies with adults, however, produced better results. According to the recent meta-analyses for adults, the estimated sensitivity and specificity of the Binax NOW S. pneumoniae assay were 74-75% and 94-97%, respectively (Table 2) [6, 81-83]. The Binax NOW S. pneumoniae assay increased the rate of etiologic diagnosis for CAP by 11-23% beyond conventional microbiological methods (Table 2) [6, 83]. In adult studies, the sensitivity of the Binax NOW S. pneumoniae assay was higher for bacteremic pneumococcal pneumonia (77-92%) than for non-bacteremic pneumococcal pneumonia (52-78%) [84-88]. Although the Binax NOW S. pneumoniae assay is less sensitive in patients with non-bacteremic pneumococcal pneumonia, it can be helpful for tailoring antibiotic therapy. The 2007 IDSA/ATS guidelines for the management of CAP recommend the use of the Binax NOW S. pneumoniae assay in the following circumstances: intensive care admission, failure of outpatient antibiotic therapy, leukopenia, active alcohol abuse, asplenia, chronic severe liver diseases, and pleural effusion [31].
One of the important advantages of the Binax NOW S. pneumoniae assay is that prior antibiotic use has less influence on the diagnostic yield. In the meta-analysis by Said et al. [6], prior antibiotic use reduced the relative diagnostic yield for blood cultures by 67% (95% confidence interval [CI], 53-77%), for sputum cultures by 34% (95% CI, 8-53%), and for the Binax NOW S. pneumoniae assay only by 26% (95% CI, 0-44%). However, the Binax NOW S. pneumoniae assay has some limitations. First, false negative results may occur in relation to low levels of the C-polysaccharide antigen [83]. Second, false positive results can be induced by cross-reaction with viridans species, asymptomatic nasopharyngeal colonization of pneumococci, and previous pneumococcal infections. Detectable amounts of teichoic acid excretion persist in 40-50% of patients' urine samples for more than 1 month following pneumococcal illness [64, 83]. If concentrated urine samples are used, the C-polysaccharide antigen can be detected in 70% of urine samples at 1 month after pneumococcal infection [89]. Also, one should be aware that a recent pneumococcal vaccination may produce a false-positive Binax NOW result [90].
Since capsular polysaccharide was first detected in the urine of patients with pneumococcal pneumonia in 1917 [91], there has been considerable interest in this detection method. Using enzyme-linked immunosorbent assay (ELISA) for the capsule, Schaffner et al. [92] found the capsular polysaccharide levels in the urine of these patients to be variable (> 500-2.5 ng/mL). In addition to depending on the severity of infection, the levels were dependent on serotypes since the serotype-specific clearance rates varied more than 250 fold [92].
Although the latex agglutination test was initially developed to detect urinary capsular polysaccharide of S. pneumoniae, the test was of limited usefulness because it was not easy to perform and could not detect all the different capsule types [13]. However, the situation recently changed with the introduction of a multiplexed immunoassay system based on the Luminex® system and monoclonal antibodies [93, 94]. Although clinical experience is limited, such multiplexed serotype-specific urinary antigen detection (UAD) assays showed excellent sensitivity (79-97%) and specificity (99-100%) for the diagnosis of bacteremic pneumococcal pneumonia based on the capsule types included in the test [94, 95].
In addition, the serotype-specific UAD assays generate additional information that allows the identification of the pneumococcal serotype causing CAP. When Bewick et al. [93] evaluated the pneumococcal serotype distribution among patients with non-invasive CAP in the U.K.; PCV13-associated serotypes were identified in 57.4% of the cases. When Sherwin et al. [96] estimated the serotype distribution of pneumococcal pneumonia using serotype-specific UAD in U.S. adults aged ≥ 50 years; PCV13-associated serotypes were detected in 80% of patients with S. pneumoniae-positive CAP or healthcare-associated pneumonia. However, since the aforementioned UAD assays were developed by vaccine companies to study the efficacy of conjugate vaccine against pneumonia, the current UAD assays cover only a limited number of serotypes (13-14 serotypes) and are not commercially available.
Similar to the Binax NOW S. pneumoniae assay, multiplex immunoassays for capsular polysaccharides were also used to directly detect the serotypes of pneumococcal empyema [97, 98]. Recently, Yu et al. [65] determined pneumococcal serotypes directly from pleural fluids using a multiplex serotype-specific immunoassay covering a relatively large number (36 types) of serotypes. This study found that pneumococcal empyema was associated with serotypes 1, 3, 7F/7A, and 19A, which are serotypes that were not covered by the 7-valent conjugate vaccine [65]. The clinical usefulness of these assays is still limited because the assays cover only a limited number of serotypes. However, even an assay with limited serotypes may be useful as the diagnostic yield of conventional microbiological methods is poor with pleural fluids.
As nucleic acid amplification tests (NAAT) using the polymerase chain reaction (PCR) became popular, it was hoped that NAAT would yield a sensitive molecular diagnostic test of pneumococcal pneumonia. So far, the use of NAAT for pneumococcal pneumonia focused on multiple genetic targets in blood and respiratory tract samples. The pneumolysin gene (ply), autolysin gene (lytA), pneumococcal surface adhesin A gene (psaA), wzg/cpsA, and the Spn9802 gene fragment have been used as PCR targets to detect S. pneumoniae [99]. The current experiences with these samples are described below.
Initially, the ply gene was widely used for the detection of S. pneumoniae. Compared with blood culture results, several studies detected S. pneumoniae DNA in blood samples using the ply PCR, with sensitivities ranging from 35 to 100% [88, 100-103]. The first challenging issue for the ply PCR test is the inability to differentiate S. pneumoniae from other streptococcus species. This poor specificity occurs because the ply gene is also present in viridans group streptococci [104, 105]. Considering the low specificity of the ply PCR, the use of PCR to detect the lytA gene was introduced and was found to have a higher specificity [22, 104, 106]. Regardless of carrier status, the lytA gene was not detected in the blood of healthy subjects [107]. Although the lytA gene is present in both S. pneumoniae and S. mitis, lytA gene sequences vary more among streptococcus species than among S. pneumoniae strains [22]. Thus, the use of lytA PCR with an appropriately designed primer may have a high sensitivity and specificity for the detection of S. pneumoniae. Noticeably, some (less than 2%) clinical isolates of S. pneumoniae are bile-insoluble and produce negative results for lytA PCR due to the alteration of the gene sequence [22]. Similar to the lytA PCR, the Spn9802 PCR is highly specific for S. pneumoniae, but it may also be positive for S. pseudopneumoniae [108]. In addition to the target genes themselves, the part of the gene amplified by PCR is very important because of allelic variation between closely related species [109]. A few studies quantitated the blood pneumococcal load by real-time PCR, and high bacteremic DNA load was associated with increased mortality [110, 111]. Thus, quantitative real-time PCR may be predictive of pneumonia severity.
When PCR targeting the ply gene was applied to lower respiratory tract specimens from patients with pneumococcal pneumonia, sensitivities ranged from 68 to 100% [112-115], but specificities were quite poor. Moreover, ply PCR positive rates for throat swab specimens were almost the same in both patients with pneumonia (55%) and in control subjects (58%) [113]. In addition to the poor specificity of the ply PCR, this difference might occur because non-quantitative PCR tests could not distinguish between true pneumococcal pneumonia and nasopharyngeal colonization. To overcome this limitation, quantitative real-time PCR has been introduced with better specific gene targets. Spn9802 real-time quantitative PCR showed 71% sensitivity and 100% specificity for the diagnosis of pneumococcal pneumonia at a cutoff of >104 copies/mL [108]. Similarly, in a study by Albrich et al. [116], lytA quantitative real-time PCR had a sensitivity of 82.2% and a specificity of 92.0% at a density of ≥ 8,000 copies/mL for distinguishing pneumococcal CAP from asymptomatic colonization. The proportion of CAP cases attributable to pneumococcus increased from 27.1 to 52.5% using that cutoff [116]. Thus, quantitative real-time PCR of respiratory specimens may be a promising diagnostic method for pneumococcal pneumonia.
Epidemiologic studies investigating antibody levels in serum samples obtained at both acute and convalescent phases of pneumococcal pneumonia should reveal the presence of the disease. Such serologic studies used diverse pneumococcal antigens, which include pneumolysin, C-polysaccharide, capsular polysaccharides, and PsaA, a highly immunogenic lipoprotein. Compared with blood culture, IgG antibody to PsaA showed good sensitivity (85-89%) and specificity (83-98%) for pneumococcal pneumonia diagnosis in Kenyan adults [117, 118]. However, the estimated sensitivity (42%) was insufficient in children [119]. Moreover, PsaA is also present in the cell walls of viridans group streptococci [120]. As the method for measuring the level of antibodies to pneumococcal capsule has been standardized by the World Health Organization (WHO) [121], Tuerlinckx et al. [122] used the assay to prospectively evaluate the etiology of CAP in children. IgG ELISA detected pneumococcal pneumonia in 80% of cases in which the diagnosis of pneumococcal pneumonia was proven with positive blood or pleural fluid culture. Although serologic tests are not affected by prior antibiotic exposure and do not require isolating bacteria, they have the potential to detect antibodies against colonized pneumococci and are not thus routinely available in clinical diagnostic laboratories. Thus, the serologic tests are primarily useful in epidemiological surveillance studies and have limited value in clinical practice.
The concentrations of acute-phase reactants increase in response to infection, inflammation, and tissue injury, so they may be useful as biomarkers to distinguish bacterial infection from non-infectious conditions [123] or to predict prognosis or therapeutic options. An advantage of biomarkers is that the test results can be produced rapidly. Such biomarkers include CRP, procalcitonin and triggering receptor expressed on myeloid cells (TREM-1). TREM-1 is a member of the immunoglobulin superfamily, is up-regulated by microbial products [124] and may stimulate secretion of several cytokines and chemokines [125]. The serum soluble TREM-1 was associated with bacteremic CAP [125] while soluble TREM-1 in bronchoalveolar lavage fluid was associated with bacterial pneumonia [124]. Procalcitonin is produced by the parafollicular cells of the thyroid and by the neuroendocrine cells of the lungs and intestines in response to pro-inflammatory stimuli, particularly stimuli of bacterial origin. Procalcitonin may become down-regulated in the presence of viral infections [126]. The level of CRP, an acute-phase protein synthesized by the liver, can rapidly increase during acute infections or inflammations. CRP binds to phosphocholine residue on pneumococcal teichoic acid or lipoteichoic acid and can activate the complement cascade [127].
At present, there are substantial clinical data with C-reactive protein (CRP) and procalcitonin. Studies of pneumococcal vaccines have shown that a serum CRP value of > 120 mg/L and a procalcitonin level of > 5 ng/mL would be useful to identify cases of pneumococcal pneumonia in children with non-specific changes on chest X-rays [128, 129]. Elevated procalcitonin levels were well correlated with positive PCR, serology, and chest X-rays but not with positive urinary Binax NOW S. pneumoniae assays in children [109]. Galetto-Lacour et al. [130] also evaluated procalcitonin and CRP as predictors of a pneumococcal etiology of CAP in hospitalized children. Elevated procalcitonin and CRP values were strongly associated with pneumococcal CAP. The sensitivity was 94.4% for procalcitonin (cutoff: 1.5 ng/mL) and 91.9% for CRP (cutoff: 100 mg/L). A procalcitonin level of ≥ 1.5 ng/mL combined with the detection of positive pneumococcal urinary antigens had a diagnostic probability for pneumococcal CAP of almost 80% (positive likelihood ratio: 4.59).
Similar to the results from pediatric studies, both procalcitonin and CRP tests were also useful in distinguishing bacterial pneumonia (including pneumococcal pneumonia) from viral pneumonia in adults [131]. Moreover, procalcitonin levels were significantly higher in adult patients with pneumococcal pneumonia than in those with other bacterial pneumonias [132] and correlated with the severity of the pneumonia [133]. For these reasons, some have suggested the inclusion of quantitative assays of CRP and procalcitonin when designing clinical trials to estimate vaccine efficacy or to study the benefit of antibiotic therapy [126, 134]. However, these acute-phase reactants cannot be used as the sole determinant when distinguishing between viral and bacterial causes of CAP [33]. Yet, when combined with other pneumococcal detection methods, biomarkers may significantly enhance the specificity of diagnosis for pneumococcal pneumonia.
The ability to rapidly and accurately diagnose pneumococcal pneumonia would improve our ability to provide appropriate therapy, assess vaccine effectiveness, and estimate the disease burden. Yet, diagnosis of pneumococcal pneumonia remains challenging. Microbiologic studies of lung tissue or bronchoalveolar lavage (BAL) fluids (yielding ≥103 cfu/mL) may be the "gold standard" for CAP diagnosis, but they are too invasive to be routinely used in clinical settings. A definitive diagnosis of pneumococcal pneumonia can be established if S. pneumoniae can be isolated from blood or pleural fluid of pneumonia patients [135]. However, their culture is often negative. Positive microscopic examination and culture of high-quality sputum samples provide strong evidence for pneumococcal pneumonia [99]. However, one must be aware that the results of such tests can depend on the quality of the specimen and that positive results may be confused with normal carriage.
Recently, an assay for teichoic acid (the Binax NOW assay) has been adopted as a part of diagnostic criteria. If an adult patient is positive in both the Binax NOW assay and microbiologic studies of high-quality sputum samples, the combined results would provide the physician with solid evidence for pneumococcal pneumonia. A procalcitonin test may also be adopted as a part of diagnostic criteria. Although not yet commercially available, the serotype-specific UAD assay seems to be more sensitive and specific than the Binax NOW S. pneumoniae assay [94, 96]. Thus, serotype-specific UAD may be a promising option for the diagnosis of pneumococcal pneumonia. In addition, quantitative real-time PCRs of blood and lower respiratory tract specimens appear to be sensitive and specific for pneumococcal pneumonia [108, 116]. Taken together, while there is a no magic test, the increased number of sensitive and specific tests should help clinicians diagnose pneumococcal pneumonia.
In the future, additional diagnostic options may include use of metabolomics approaches such as analysis of urinary metabolites with nuclear magnetic resonance (NMR) [136] or analyzing patients' breath or bacterial isolates with mass spectrometry. A breath test is an attractive diagnostic option for pneumococcal pneumonia; indeed, volatile metabolites are being investigated as biomarkers for specific bacterial pathogens, but, at present, data are quite insufficient to determine if such a test would be sufficiently sensitive and specific [66]. On the other hand, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) is used in many clinical laboratories to identify bacteria. Although MALDI-TOF-based systems have been found to be easy-to-use, cost-effective, and fast with high-throughput performances, they have shown limitations in discriminating between S. pneumoniae and the viridians group [137, 138]. However, rapid improvements are being made and Vitek MS MALDI-TOF mass spectrometry system has been reported to perform as well as the conventional identification method (e.g., optochin test) [139].
Although S. pneumoniae is the most common etiologic agent for CAP, Haemophilus influenzae, S. aureus, Klebsiella pneumoniae, and atypical pathogens (Mycoplasma pneumoniae and respiratory viruses) are also important causes of CAP. In clinical practice, therefore, it is desirable to have a diagnostic test for these pathogens as well. Some commercial multiplexed NAATs are designed to detect the DNA of these pathogens. Park et al. [140] evaluated a multiplexed NAAT designed to detect six respiratory bacterial pathogens (S. pneumoniae, H. influenzae, M. pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, and Bordetella pertussis) in children. The agreement rates between multiplex PCR and cultures for S. pneumoniae and H. influenzae were 92.9% and 91.1%, respectively. However, S. pneumoniae and H. influenzae can exist as commensal organisms of the upper respiratory tract, so quantitative multiplex NAAT would be required to better detect and differentiate the etiologic agent of CAP. Indeed, a quantitative multiplex PCR to detect S. pneumoniae, H. influenzae, and Neisseria meningitidis was described in 2010 [141]. Multiplex assays for all these pathogens should potentially revolutionize both the diagnosis of pneumonia and its treatments in the future.
References
1. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004; 18:761–776. PMID: 15555823.

2. Marrie TJ, Huang JQ. Epidemiology of community-acquired pneumonia in Edmonton, Alberta: an emergency department-based study. Can Respir J. 2005; 12:139–142. PMID: 15875065.

3. Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996; 275:134–141. PMID: 8531309.

4. Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, Phan HM, Solomon E. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000; 79:210–221. PMID: 10941350.

5. Yoo KH, Yoo CG, Kim SK, Jung JY, Lee MG, Uh ST, Shim TS, Jeon K, Shim JJ, Lee HB, Chung CR, Kang KW, Jung KS. Economic burden and epidemiology of pneumonia in Korean adults aged over 50 years. J Korean Med Sci. 2013; 28:888–895. PMID: 23772154.

6. Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, Andreo F, Beovic B, Blanco S, Boersma WG, Boulware DR, Butler JC, Carratalà J, Chang FY, Charles PG, Diaz AA, Domínguez J, Ehara N, Endeman H, Falcó V, Falguera M, Fukushima K, Garcia-Vidal C, Genne D, Guchev IA, Gutierrez F, Hernes SS, Hoepelman AI, Hohenthal U, Johansson N, Kolek V, Kozlov RS, Lauderdale TL, Mareković I, Masiá M, Matta MA, Miró Ò, Murdoch DR, Nuermberger E, Paolini R, Perelló R, Snijders D, Plečko V, Sordé R, Strålin K, van der Eerden MM, Vila-Corcoles A, Watt JP. AGEDD Adult Pneumococcal Burden Study Team. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013; 8:e60273. PMID: 23565216.

7. Briles DE. Protection of the elderly from pneumococcal pneumonia with a protein-based vaccine? Mech Ageing Dev. 2004; 125:129–131. PMID: 15037017.

8. Calix JJ, Nahm MH. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J Infect Dis. 2010; 202:29–38. PMID: 20507232.
9. Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995; 33:2759–2762. PMID: 8567920.
10. Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011; 331:430–434. PMID: 21273480.

11. Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci. 2013; 28:4–15. PMID: 23341706.

12. Hill PC, Akisanya A, Sankareh K, Cheung YB, Saaka M, Lahai G, Greenwood BM, Adegbola RA. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian villagers. Clin Infect Dis. 2006; 43:673–679. PMID: 16912937.
13. Werno AM, Murdoch DR. Medical microbiology: laboratory diagnosis of invasive pneumococcal disease. Clin Infect Dis. 2008; 46:926–932. PMID: 18260752.
14. Dyson C, Barnes RA, Harrison GA. Infective endocarditis: an epidemiological review of 128 episodes. J Infect. 1999; 38:87–93. PMID: 10342647.

15. Keith ER, Podmore RG, Anderson TP, Murdoch DR. Characteristics of Streptococcus pseudopneumoniae isolated from purulent sputum samples. J Clin Microbiol. 2006; 44:923–927. PMID: 16517877.
16. Dilworth JA, Stewart P, Gwaltney JM Jr, Hendley JO, Sande MA. Methods to improve detection of pneumococci in respiratory secretions. J Clin Microbiol. 1975; 2:453–455. PMID: 421.

17. Sondag JE, Morgens RK, Hoppe JE, Marr JJ. Detection of pneumococci in respiratory secretions: clinical evaluation of gentamicin blood agar. J Clin Microbiol. 1977; 5:397–400. PMID: 16034.

18. Wu SC, Trask LM, Phee RE. Comparison of media and culture techniques for detection of Streptococcus pneumoniae in respiratory secretions. J Clin Microbiol. 1980; 12:772–775. PMID: 7031078.
19. Schmid RE, Washington JA 2nd, Anhalt JP. Gentamicin-blood agar for isolation of Streptococcus pneumoniae from respiratory secretions. J Clin Microbiol. 1978; 7:426–427. PMID: 26693.
20. Arbique JC, Poyart C, Trieu-Cuot P, Quesne G, Carvalho Mda G, Steigerwalt AG, Morey RE, Jackson D, Davidson RJ, Facklam RR. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J Clin Microbiol. 2004; 42:4686–4696. PMID: 15472328.
21. Kellogg JA, Bankert DA, Elder CJ, Gibbs JL, Smith MC. Identification of Streptococcus pneumoniae revisited. J Clin Microbiol. 2001; 39:3373–3375. PMID: 11526182.
22. Greve T, Møller JK. Accuracy of using the lytA gene to distinguish Streptococcus pneumoniae from related species. J Med Microbiol. 2012; 61:478–482. PMID: 22135022.
23. Suzuki N, Yuyama M, Maeda S, Ogawa H, Mashiko K, Kiyoura Y. Genotypic identification of presumptive Streptococcus pneumoniae by PCR using four genes highly specific for S. pneumoniae. J Med Microbiol. 2006; 55:709–714. PMID: 16687588.
24. Leegaard TM, Bootsma HJ, Caugant DA, Eleveld MJ, Mannsåker T, Frøholm LO, Gaustad P, Høiby EA, Hermans PW. Phenotypic and genomic characterization of pneumococcus-like streptococci isolated from HIV-seropositive patients. Microbiology. 2010; 156:838–848. PMID: 19959577.

25. Park IH, Kim KH, Andrade AL, Briles DE, McDaniel LS, Nahm MH. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. MBio. 2012; 3:pii: e00035-12.

26. Hanage WP, Kaijalainen T, Herva E, Saukkoriipi A, Syrjanen R, Spratt BG. Using multilocus sequence data to define the pneumococcus. J Bacteriol. 2005; 187:6223–6230. PMID: 16109964.

27. Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, Weber GF, Petrillo MK, Houck PM, Fine JM. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997; 278:2080–2084. PMID: 9403422.

28. Jadavji T, Law B, Lebel MH, Kennedy WA, Gold R, Wang EE. A practical guide for the diagnosis and treatment of pediatric pneumonia. CMAJ. 1997; 156:S703–S711. PMID: 9068582.
29. Cardoso MR, Nascimento-Carvalho CM, Ferrero F, Alves FM, Cousens SN. Adding fever to WHO criteria for diagnosing pneumonia enhances the ability to identify pneumonia cases among wheezing children. Arch Dis Child. 2011; 96:58–61. PMID: 20870628.

30. Irfan M, Farooqi J, Hasan R. Community-acquired pneumonia. Curr Opin Pulm Med. 2013; 19:198–208. PMID: 23422417.

31. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious Diseases Society of America. American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44(Suppl 2):S27–S72. PMID: 17278083.

32. Anevlavis S, Bouros D. Community acquired bacterial pneumonia. Expert Opin Pharmacother. 2010; 11:361–374. PMID: 20085502.

33. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011; 53:e25–e76. PMID: 21880587.

34. Scott JA, Marston EL, Hall AJ, Marsh K. Diagnosis of pneumococcal pneumonia by psaA PCR analysis of lung aspirates from adult patients in Kenya. J Clin Microbiol. 2003; 41:2554–2559. PMID: 12791880.
35. Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995; 333:1618–1624. PMID: 7477199.

36. Barrett-Connor E. The nonvalue of sputum culture in the diagnosis of pneumococcal pneumonia. Am Rev Respir Dis. 1971; 103:845–848. PMID: 4397407.
37. Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004; 39:165–169. PMID: 15307023.

38. Murray PR, Washington JA. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin Proc. 1975; 50:339–344. PMID: 1127999.
39. Reed WW, Byrd GS, Gates RH Jr, Howard RS, Weaver MJ. Sputum Gram's stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996; 165:197–204. PMID: 8987424.
40. Anevlavis S, Petroglou N, Tzavaras A, Maltezos E, Pneumatikos I, Froudarakis M, Anevlavis E, Bouros D. A prospective study of the diagnostic utility of sputum Gram stain in pneumonia. J Infect. 2009; 59:83–89. PMID: 19564045.

41. Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000; 31:869–874. PMID: 11049763.

42. Miyashita N, Shimizu H, Ouchi K, Kawasaki K, Kawai Y, Obase Y, Kobashi Y, Oka M. Assessment of the usefulness of sputum Gram stain and culture for diagnosis of community-acquired pneumonia requiring hospitalization. Med Sci Monit. 2008; 14:CR171–CR176. PMID: 18376343.
43. Fine MJ, Orloff JJ, Rihs JD, Vickers RM, Kominos S, Kapoor WN, Arena VC, Yu VL. Evaluation of housestaff physicians' preparation and interpretation of sputum Gram stains for community-acquired pneumonia. J Gen Intern Med. 1991; 6:189–198. PMID: 1712384.

44. Fiala M. A study of the combined role of viruses, mycoplasmas and bacteria in adult pneumonia. Am J Med Sci. 1969; 257:44–51. PMID: 4387669.

45. Tempest B, Morgan R, Davidson M, Eberle B, Oseasohn R. The value of respiratory tract bacteriology in pneumococcal pneumonia among Navajo Indians. Am Rev Respir Dis. 1974; 109:577–578. PMID: 4150886.
46. Davidson M, Tempest B, Palmer DL. Bacteriologic diagnosis of acute pneumonia. Comparison of sputum, transtracheal aspirates, and lung aspirates. JAMA. 1976; 235:158–163. PMID: 521.

47. Drew WL. Value of sputum culture in diagnosis of pneumococcal pneumonia. J Clin Microbiol. 1977; 6:62–65. PMID: 18489.

48. Kalin M. Bacteremic pneumococcal pneumonia: value of culture of nasopharyngeal specimens and examination of washed sputum specimens. Eur J Clin Microbiol. 1982; 1:394–396. PMID: 7160371.

49. Guzzetta P, Toews GB, Robertson KJ, Pierce AK. Rapid diagnosis of community-acquired bacterial pneumonia. Am Rev Respir Dis. 1983; 128:461–464. PMID: 6412607.

50. Perlino CA. Laboratory diagnosis of pneumonia due to Streptococcus pneumoniae. J Infect Dis. 1984; 150:139–144. PMID: 6611379.
51. Watanakunakorn C, Bailey TA. Adult bacteremic pneumococcal pneumonia in a community teaching hospital, 1992-1996. A detailed analysis of 108 cases. Arch Intern Med. 1997; 157:1965–1971. PMID: 9308508.

52. Torres JM, Cardenas O, Vasquez A, Schlossberg D. Streptococcus pneumoniae bacteremia in a community hospital. Chest. 1998; 113:387–390. PMID: 9498956.
53. Chalasani NP, Valdecanas MA, Gopal AK, McGowan JE Jr, Jurado RL. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995; 108:932–936. PMID: 7555163.

54. Fang GD, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W, Grayston JT, Wang SP, Kohler R, Muder RR. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine (Baltimore). 1990; 69:307–316. PMID: 2205784.
55. Lieberman D, Schlaeffer F, Boldur I, Horowitz S, Friedman MG, Leiononen M, Horovitz O, Manor E, Porath A. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax. 1996; 51:179–184. PMID: 8711652.

56. Luna CM, Famiglietti A, Absi R, Videla AJ, Nogueira FJ, Fuenzalida AD, Gené RJ. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest. 2000; 118:1344–1354. PMID: 11083685.
57. Petti CA, Woods CW, Reller LB. Streptococcus pneumoniae antigen test using positive blood culture bottles as an alternative method to diagnose pneumococcal bacteremia. J Clin Microbiol. 2005; 43:2510–2512. PMID: 15872298.
58. Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003; 22:499–504. PMID: 12799505.

59. Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006; 25:250–254. PMID: 16511389.

60. Gupta R, Crowley S. Increasing paediatric empyema admissions. Thorax. 2006; 61:179–180. PMID: 16443710.

61. Langley JM, Kellner JD, Solomon N, Robinson JL, Le Saux N, McDonald J, Ulloa-Gutierrez R, Tan B, Allen U, Dobson S, Joudrey H. Empyema associated with community-acquired pneumonia: a Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis. 2008; 8:129. PMID: 18816409.

62. Burgos J, Falcó V, Pahissa A. The increasing incidence of empyema. Curr Opin Pulm Med. 2013; 19:350–356. PMID: 23508113.

63. Holmberg H, Krook A. Comparison of enzyme-linked immunosorbent assay with coagglutination and latex agglutination for rapid diagnosis of pneumococcal pneumonia by detecting antigen in sputa. Eur J Clin Microbiol. 1986; 5:282–286. PMID: 3743553.

64. Andreo F, Prat C, Ruiz-Manzano J, Lores L, Blanco S, Cuesta MA, Giménez M, Domínguez J. Persistence of Streptococcus pneumoniae urinary antigen excretion after pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis. 2009; 28:197–201. PMID: 18830727.
65. Yu J, Salamon D, Marcon M, Nahm MH. Pneumococcal serotypes causing pneumonia with pleural effusion in pediatric patients. J Clin Microbiol. 2011; 49:534–538. PMID: 21123535.

66. Bos LD, Sterk PJ, Schultz MJ. Volatile metabolites of pathogens: a systematic review. PLoS Pathog. 2013; 9:e1003311. PMID: 23675295.

67. Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun. 2008; 76:2767–2776. PMID: 18391008.
68. Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun. 2006; 74:1171–1180. PMID: 16428766.
69. Charkaluk ML, Kalach N, Mvogo H, Dehecq E, Magentie H, Raymond J, Gendrel D, Kremp O, Decoster A. Assessment of a rapid urinary antigen detection by an immunochromatographic test for diagnosis of pneumococcal infection in children. Diagn Microbiol Infect Dis. 2006; 55:89–94. PMID: 16530375.

70. Domínguez J, Blanco S, Rodrigo C, Azuara M, Galí N, Mainou A, Esteve A, Castellví A, Prat C, Matas L, Ausina V. Usefulness of urinary antigen detection by an immunochromatographic test for diagnosis of pneumococcal pneumonia in children. J Clin Microbiol. 2003; 41:2161–2163. PMID: 12734268.

71. Dowell SF, Garman RL, Liu G, Levine OS, Yang YH. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin Infect Dis. 2001; 32:824–825. PMID: 11229853.

72. Elemraid MA, Rushton SP, Clark JE, Perry JD, Thomas MF, Sails AD, Gennery AR, Spencer DA. on behalf of the North East of England Paediatric Respiratory Infection Study Group. A case-control study to assess the urinary pneumococcal antigen test in childhood pneumonia. Clin Pediatr (Phila). 2013; [In press].

73. Hamann L, Stamme C, Ulmer AJ, Schumann RR. Inhibition of LPS-induced activation of alveolar macrophages by high concentrations of LPS-binding protein. Biochem Biophys Res Commun. 2002; 295:553–560. PMID: 12150986.

74. Strachan RE, Cornelius A, Gilbert GL, Gulliver T, Martin A, McDonald T, Nixon GM, Roseby R, Ranganathan S, Selvadurai H, Smith G, Soto-Martinez M, Suresh S, Teoh L, Thapa K, Wainwright CE, Jaffé A. Australian Research Network in Empyema (ARNiE). A bedside assay to detect Streptococcus pneumoniae in children with empyema. Pediatr Pulmonol. 2011; 46:179–183. PMID: 20963842.
75. Porcel JM, Ruiz-González A, Falguera M, Nogués A, Galindo C, Carratalá J, Esquerda A. Contribution of a pleural antigen assay (Binax NOW) to the diagnosis of pneumococcal pneumonia. Chest. 2007; 131:1442–1447. PMID: 17317736.

76. Picazo JJ, Contreras JR, Ríos E, Culebras E, Rodríguez-Avial I, Méndez C, Betriu C. Heracles Study Group. Rapid diagnosis of invasive pneumococcal disease in pediatric population. J Microbiol Methods. 2013; 93:116–120. PMID: 23499921.

77. Martinón-Torres F, Dosil-Gallardo S, Perez del Molino-Bernal ML, Sánchez FP, Tarrago D, Alvez F, Diaz SP, Martinón-Torres N, Martinon Sanchez JM. Pleural antigen assay in the diagnosis of pediatric pneumococcal empyema. J Crit Care. 2012; 27:321.e1–321.e4. PMID: 21737239.

78. Lee JH, Kim SH, Lee J, Choi EH, Lee HJ. Diagnosis of pneumococcal empyema using immunochromatographic test on pleural fluid and serotype distribution in Korean children. Diagn Microbiol Infect Dis. 2012; 72:119–124. PMID: 22079140.

79. Casado Flores J, Nieto Moro M, Berrón S, Jiménez R, Casal J. Usefulness of pneumococcal antigen detection in pleural effusion for the rapid diagnosis of infection by Streptococcus pneumoniae. Eur J Pediatr. 2010; 169:581–584. PMID: 19806363.
80. Le Monnier A, Carbonnelle E, Zahar JR, Le Bourgeois M, Abachin E, Quesne G, Varon E, Descamps P, De Blic J, Scheinmann P, Berche P, Ferroni A. Microbiological diagnosis of empyema in children: comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006; 42:1135–1140. PMID: 16575731.

81. Sinclair A, Xie X, Teltscher M, Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 2013; 51:2303–2310. PMID: 23678060.
82. Horita N, Miyazawa N, Kojima R, Kimura N, Inoue M, Ishigatsubo Y, Kaneko T. Sensitivity and specificity of the Streptococcus pneumoniae urinary antigen test for unconcentrated urine from adult patients with pneumonia: a meta-analysis. Respirology. 2013; 18:1177–1183. PMID: 23910720.
83. Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect. 2007; 55:300–309. PMID: 17692384.

84. Smith MD, Derrington P, Evans R, Creek M, Morris R, Dance DA, Cartwright K. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003; 41:2810–2813. PMID: 12843005.
85. Rosón B, Fernández-Sabé N, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis. 2004; 38:222–226. PMID: 14699454.

86. Murdoch DR, Laing RT, Mills GD, Karalus NC, Town GI, Mirrett S, Reller LB. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001; 39:3495–3498. PMID: 11574562.
87. Gutiérrez F, Masiá M, Rodríguez JC, Ayelo A, Soldán B, Cebrián L, Mirete C, Royo G, Hidalgo AM. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis. 2003; 36:286–292. PMID: 12539069.
88. Domínguez J, Galí N, Blanco S, Pedroso P, Prat C, Matas L, Ausina V. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest. 2001; 119:243–249. PMID: 11157611.
89. Marcos MA, Jiménez de Anta MT, de la Bellacasa JP, González J, Martínez E, García E, Mensa J, de Roux A, Torres A. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003; 21:209–214. PMID: 12608431.

90. Navarro D, García-Maset L, Gimeno C, Escribano A, García-de-Lomas J. Spanish Pneumococcal Infection Study Network. Performance of the Binax NOW Streptococcus pneumoniae urinary antigen assay for diagnosis of pneumonia in children with underlying pulmonary diseases in the absence of acute pneumococcal infection. J Clin Microbiol. 2004; 42:4853–4855. PMID: 15472361.
91. Dochez AR, Avery OT. The elaboration of specific soluble substance by pneumococcus during growth. J Exp Med. 1917; 26:477–493. PMID: 19868163.

92. Schaffner A, Michel-Harder C, Yeginsoy S. Detection of capsular polysaccharide in serum for the diagnosis of pneumococcal pneumonia: clinical and experimental evaluation. J Infect Dis. 1991; 163:1094–1102. PMID: 1826918.

93. Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012; 67:540–545. PMID: 22374921.

94. Pride MW, Huijts SM, Wu K, Souza V, Passador S, Tinder C, Song E, Elfassy A, McNeil L, Menton R, French R, Callahan J, Webber C, Gruber WC, Bonten MJ, Jansen KU. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol. 2012; 19:1131–1141. PMID: 22675155.
95. Sheppard CL, Harrison TG, Smith MD, George RC. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J Med Microbiol. 2011; 60:49–55. PMID: 20864547.
96. Sherwin RL, Gray S, Alexander R, McGovern PC, Graepel J, Pride MW, Purdy J, Paradiso P, File TM Jr. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis. 2013; 208:1813–1820. PMID: 24092845.
97. Eastham KM, Freeman R, Kearns AM, Eltringham G, Clark J, Leeming J, Spencer DA. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004; 59:522–525. PMID: 15170039.

98. Fletcher M, Leeming J, Cartwright K, Finn A. South West of England Invasive Community Acquired Infection Study Group. Childhood empyema: limited potential impact of 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2006; 25:559–560. PMID: 16732158.

99. Blaschke AJ. Interpreting assays for the detection of Streptococcus pneumoniae. Clin Infect Dis. 2011; 52(Suppl 4):S331–S337. PMID: 21460292.
100. Lorente ML, Falguera M, Nogués A, González AR, Merino MT, Caballero MR. Diagnosis of pneumococcal pneumonia by polymerase chain reaction (PCR) in whole blood: a prospective clinical study. Thorax. 2000; 55:133–137. PMID: 10639531.

101. Rudolph KM, Parkinson AJ, Black CM, Mayer LW. Evaluation of polymerase chain reaction for diagnosis of pneumococcal pneumonia. J Clin Microbiol. 1993; 31:2661–2666. PMID: 8253962.

102. Salo P, Ortqvist A, Leinonen M. Diagnosis of bacteremic pneumococcal pneumonia by amplification of pneumolysin gene fragment in serum. J Infect Dis. 1995; 171:479–482. PMID: 7844395.

103. Sheppard CL, Harrison TG, Kearns AM, Guiver M, Creek M, Evans R, Smith MD, Eltringham G, Cartwright KA, George RC. Diagnosis of invasive pneumococcal infection by PCR amplification of Streptococcus pneumoniae genomic fragments in blood: a multi-centre comparative study. Commun Dis Public Health. 2003; 6:221–227. PMID: 14708272.
104. Carvalho Mda G, Tondella ML, McCaustland K, Weidlich L, McGee L, Mayer LW, Steigerwalt A, Whaley M, Facklam RR, Fields B, Carlone G, Ades EW, Dagan R, Sampson JS. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol. 2007; 45:2460–2466. PMID: 17537936.
105. Neeleman C, Klaassen CH, Klomberg DM, de Valk HA, Mouton JW. Pneumolysin is a key factor in misidentification of macrolide-resistant Streptococcus pneumoniae and is a putative virulence factor of S. mitis and other streptococci. J Clin Microbiol. 2004; 42:4355–4357. PMID: 15365043.
106. Abdeldaim G, Herrmann B, Mölling P, Holmberg H, Blomberg J, Olcén P, Strålin K. Usefulness of real-time PCR for lytA, ply, and Spn9802 on plasma samples for the diagnosis of pneumococcal pneumonia. Clin Microbiol Infect. 2010; 16:1135–1141. PMID: 19832718.

107. Azzari C, Cortimiglia M, Moriondo M, Canessa C, Lippi F, Ghiori F, Becciolini L, de Martino M, Resti M. Pneumococcal DNA is not detectable in the blood of healthy carrier children by real-time PCR targeting the lytA gene. J Med Microbiol. 2011; 60:710–714. PMID: 21349984.

108. Abdeldaim GM, Strålin K, Olcén P, Blomberg J, Herrmann B. Toward a quantitative DNA-based definition of pneumococcal pneumonia: a comparison of Streptococcus pneumoniae target genes, with special reference to the Spn9802 fragment. Diagn Microbiol Infect Dis. 2008; 60:143–150. PMID: 17916422.
109. Vernet G, Saha S, Satzke C, Burgess DH, Alderson M, Maisonneuve JF, Beall BW, Steinhoff MC, Klugman KP. Laboratory-based diagnosis of pneumococcal pneumonia: state of the art and unmet needs. Clin Microbiol Infect. 2011; 17(Suppl 3):1–13. PMID: 21457174.

110. Carrol ED, Guiver M, Nkhoma S, Mankhambo LA, Marsh J, Balmer P, Banda DL, Jeffers G, White SA, Molyneux EM, Molyneux ME, Smyth RL, Hart CA. IPD Study Group. High pneumococcal DNA loads are associated with mortality in Malawian children with invasive pneumococcal disease. Pediatr Infect Dis J. 2007; 26:416–422. PMID: 17468652.

111. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010; 201:32–41. PMID: 19947881.

112. Butler JC, Bosshardt SC, Phelan M, Moroney SM, Tondella ML, Farley MM, Schuchat A, Fields BS. Classical and latent class analysis evaluation of sputum polymerase chain reaction and urine antigen testing for diagnosis of pneumococcal pneumonia in adults. J Infect Dis. 2003; 187:1416–1423. PMID: 12717623.

113. Murdoch DR, Anderson TP, Beynon KA, Chua A, Fleming AM, Laing RT, Town GI, Mills GD, Chambers ST, Jennings LC. Evaluation of a PCR assay for detection of Streptococcus pneumoniae in respiratory and nonrespiratory samples from adults with community-acquired pneumonia. J Clin Microbiol. 2003; 41:63–66. PMID: 12517826.
114. Paul ML, Benn RA. Sputum PCR for the detection of pneumococcal lower respiratory tract infection. Pathology. 1997; 29:104–105. PMID: 9094192.

115. Wheeler J, Freeman R, Steward M, Henderson K, Lee MJ, Piggott NH, Eltringham GJ, Galloway A. Detection of pneumolysin in sputum. J Med Microbiol. 1999; 48:863–866. PMID: 10482298.

116. Albrich WC, Madhi SA, Adrian PV, van Niekerk N, Mareletsi T, Cutland C, Wong M, Khoosal M, Karstaedt A, Zhao P, Deatly A, Sidhu M, Jansen KU, Klugman KP. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012; 54:601–609. PMID: 22156852.

117. Tharpe JA, Russell H, Leinonen M, Plikaytis BD, Breiman RF, Carlone GM, Ades EW, Sampson JS. Comparison of a pneumococcal common protein (PsaA) antibody ELISA and a PsaA immune complex ELISA for detection of pneumococcal serum antibody. Pathobiology. 1998; 66:77–83. PMID: 9645631.

118. Scott JA, Obiero J, Hall AJ, Marsh K. Validation of immunoglobulin G enzyme-linked immunosorbent assay for antibodies to pneumococcal surface adhesin A in the diagnosis of pneumococcal pneumonia among adults in Kenya. J Infect Dis. 2002; 186:220–226. PMID: 12134258.

119. Scott JA, Mlacha Z, Nyiro J, Njenga S, Lewa P, Obiero J, Otieno H, Sampson JS, Carlone GM. Diagnosis of invasive pneumococcal disease among children in Kenya with enzyme-linked immunosorbent assay for immunoglobulin G antibodies to pneumococcal surface adhesin A. Clin Diagn Lab Immunol. 2005; 12:1195–1201. PMID: 16210483.

120. Jado I, Fenoll A, Casal J, Pérez A. Identification of the psaA gene, coding for pneumococcal surface adhesin A, in viridans group streptococci other than Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2001; 8:895–898. PMID: 11527799.
121. Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013; 19:412–425. PMID: 23657429.

122. Tuerlinckx D, Smet J, De Schutter I, Jamart J, Vergison A, Raes M, Smeesters PR, Verhaegen J, Surmont F, Malfroot A, Mascart F. Evaluation of a WHO-validated serotype-specific serological assay for the diagnosis of pneumococcal etiology in children with community-acquired pneumonia. Pediatr Infect Dis J. 2013; 32:e277–e284. PMID: 23407099.

123. Upadhyay S, Niederman MS. Biomarkers: what is their benefit in the identification of infection, severity assessment, and management of community-acquired pneumonia? Infect Dis Clin North Am. 2013; 27:19–31. PMID: 23398863.
124. Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004; 350:451–458. PMID: 14749453.

125. Ruiz-González A, Esquerda A, Falguera M, Abdulghani N, Cabezas P, Bielsa S, Porcel JM. Triggering receptor (TREM-1) expressed on myeloid cells predicts bacteremia better than clinical variables in community-acquired pneumonia. Respirology. 2011; 16:321–325. PMID: 21114709.
126. Niederman MS. Biological markers to determine eligibility in trials for community-acquired pneumonia: a focus on procalcitonin. Clin Infect Dis. 2008; 47(Suppl 3):S127–S132. PMID: 18986278.

127. Agrawal A, Simpson MJ, Black S, Carey MP, Samols D. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J Immunol. 2002; 169:3217–3222. PMID: 12218140.

128. Madhi SA, Heera JR, Kuwanda L, Klugman KP. Use of procalcitonin and C-reactive protein to evaluate vaccine efficacy against pneumonia. PLoS Med. 2005; 2:e38. PMID: 15736995.

129. Madhi SA, Klugman KP. World Health Organisation definition of "radiologically-confirmed pneumonia" may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine. 2007; 25:2413–2419. PMID: 17005301.

130. Galetto-Lacour A, Alcoba G, Posfay-Barbe KM, Cevey-Macherel M, Gehri M, Ochs MM, Brookes RH, Siegrist CA, Gervaix A. Elevated inflammatory markers combined with positive pneumococcal urinary antigen are a good predictor of pneumococcal community-acquired pneumonia in children. Pediatr Infect Dis J. 2013; 32:1175–1179. PMID: 23694836.

131. Song JY, Cheong HJ, Heo JY, Noh JY, Yong HS, Kim YK, Kang EY, Choi WS, Jo YM, Kim WJ. Clinical, laboratory and radiologic characteristics of 2009 pandemic influenza A/H1N1 pneumonia: primary influenza pneumonia versus concomitant/secondary bacterial pneumonia. Influenza Other Respir Viruses. 2011; 5:e535–e543. PMID: 21682848.

132. Horie M, Ugajin M, Suzuki M, Noguchi S, Tanaka W, Yoshihara H, Kawakami M, Kichikawa Y, Sakamoto Y. Diagnostic and prognostic value of procalcitonin in community-acquired pneumonia. Am J Med Sci. 2012; 343:30–35. PMID: 22207498.

133. Lacoma A, Rodríguez N, Prat C, Ruiz-Manzano J, Andreo F, Ramírez A, Bas A, Pérez M, Ausina V, Domínguez J. Usefulness of consecutive biomarkers measurement in the management of community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2012; 31:825–833. PMID: 21870054.

134. Klugman KP, Madhi SA, Albrich WC. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin Infect Dis. 2008; 47(Suppl 3):S202–S206. PMID: 18986290.
135. Penaranda M, Falco V, Payeras A, Jordano Q, Curran A, Pareja A, Samperiz G, Dalmau D, Ribera E, Riera M. Effectiveness of polysaccharide pneumococcal vaccine in HIV-infected patients: a case-control study. Clin Infect Dis. 2007; 45:e82–e87. PMID: 17806042.

136. Slupsky CM, Rankin KN, Fu H, Chang D, Rowe BH, Charles PG, McGeer A, Low D, Long R, Kunimoto D, Sawyer MB, Fedorak RN, Adamko DJ, Saude EJ, Shah SL, Marrie TJ. Pneumococcal pneumonia: potential for diagnosis through a urinary metabolic profile. J Proteome Res. 2009; 8:5550–5558. PMID: 19817432.

137. Stevenson LG, Drake SK, Murray PR. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010; 48:444–447. PMID: 19955282.

138. Risch M, Radjenovic D, Han JN, Wydler M, Nydegger U, Risch L. Comparison of MALDI TOF with conventional identification of clinically relevant bacteria. Swiss Med Wkly. 2010; 140:w13095. PMID: 20924806.

139. Dubois D, Segonds C, Prere MF, Marty N, Oswald E. Identification of clinical Streptococcus pneumoniae isolates among other alpha and nonhemolytic streptococci by use of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system. J Clin Microbiol. 2013; 51:1861–1867. PMID: 23576536.
140. Park J, Kim JK, Rheem I, Kim J. Evaluation of Seeplex Pneumobacter multiplex PCR kit for the detection of respiratory bacterial pathogens in pediatric patients. Korean J Lab Med. 2009; 29:307–313. PMID: 19726892.
141. Abdeldaim GM, Strålin K, Korsgaard J, Blomberg J, Welinder-Olsson C, Herrmann B. Multiplex quantitative PCR for detection of lower respiratory tract infection and meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis. BMC Microbiol. 2010; 10:310. PMID: 21129171.

Figure 1
S. pneumoniae isolates expressing most capsule types make small round colonies similar to doughnuts on blood agar plate (A) but serotype 3 and 37 pneumococci develop characteristically large mucoid colonies (B).
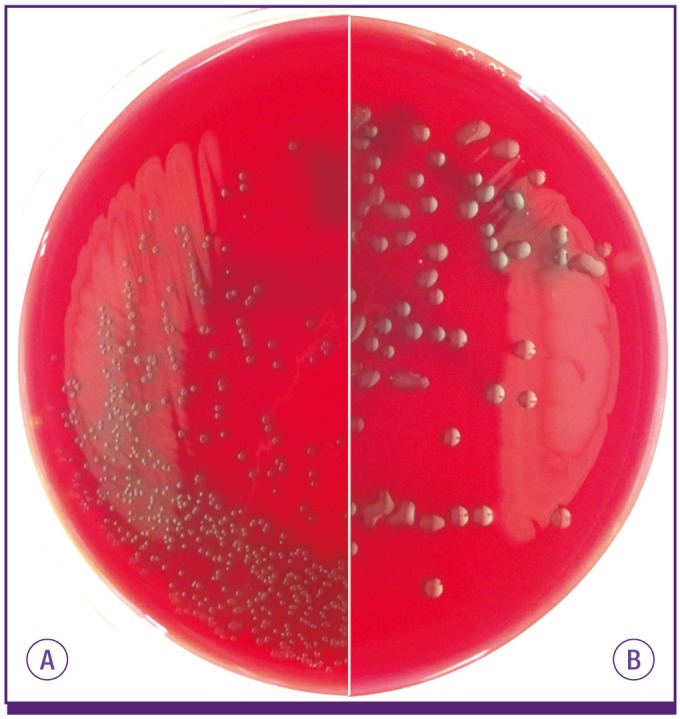
Figure 2
S. pneumoniae growth is inhibited around the paper disk containing optochin (A). The test tube containing S. pneumoniae shows a loss of turbidity in the presence of sodium deoxycholate (bile salts) due to bacterial lysis while the test tube containing viridans species is turbid (B).
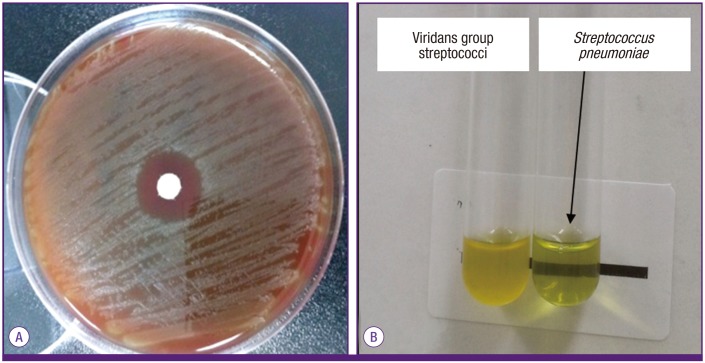
Table 1
Review of Binax NOW S. pneumoniae assay performance for the diagnosis of pneumococcal empyema




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download