Abstract
Background
Plasmodium vivax malaria is an acute debilitating illness characterized by recurrent paroxysmal fever and relapses from hypnozoites in the liver. Although a few studies reported clinical characteristics of vivax malaria in civilians after reemergence in the Republic of Korea, only a small group of patients was analyzed.
Materials and Methods
We retrospectively reviewed the medical records of patients who had been diagnosed with vivax malaria by peripheral blood smear in a university-affiliated hospital located in a malaria-endemic area between January 2005 and December 2009.
Results
During the study period, a total of 352 malarial cases from 341 patients were diagnosed. Vivax malaria was most commonly developed in July and August, 24.7% (87/352), and 21.9% (77/352), respectively. The mean (SD) age was 42.5 (14.7) years and the number of male patients was 243 (71.3%). Six patients had a previous history of vivax malaria from 6 months to 10 years before. A total of 337 patients (98.8%) had fever and the mean (SD) body temperature was 38.3 (1.4)℃. Common associated symptoms were chills (213/341, 62.5%), headache (115/341, 33.7%), and myalgia (85/341, 24.9%). Laboratory findings included thrombocytopenia (340/341, 99.7%), anemia (97/341, 28.5%), leukopenia (148/341, 43.4%), increase of aspartate transaminase (177/341, 51.9%), and increase of alanine transaminase (187/341, 54.8%). Hypotension (14/341, 4.1%), altered mentality (3/341, 0.9%), azotemia (3/341, 0.9%), spleen infarction (2/341, 0.6%), and spleen rupture (1/341, 0.3%) developed as complications. Chloroquine was administered to all patients and primaquine was administered with mean (SD) 3.39 (0.82) mg/kg to 320 patients. There were 11 recurrent infections during the study period. The median (range) time to recurrent infection was 100 (32-285) days. Platelet counts were higher (86,550 vs. 56,910/mm3) and time to treatment of malaria was shorter (5 vs. 7 days) in relapsed cases compared with first occurrence cases (P=0.046).
Conclusions
The overall recurrence rate of vivax malaria was 3.2% (11/341) in this study. In recurred cases, malaria was diagnosed earlier and thrombocytopenia was less severe. To evaluate the risk factors associated with recurrence and adequate dose of primaquine in Korean patients, further large-scale prospective studies will be needed.
Malaria had disappeared in the Republic of Korea, but it has re-emerged since 1993. Until the late 1990s, it was mostly found in soldiers on active duty. However, the rate of civilians infected gradually increased, accounting for more than half of all malaria patients since 2002 [1]. In the beginning of its re-emergence, malaria occurred mostly in areas such as Paju and Yunchun, but recently it has been more prevalent in the westerm parts of Gyeonggi-do such as Ganghwa County, Gimpo, Incheon, Paju, and Goyangi [2].
Indigenous vivax malaria is not a common cause of death, but a recurrence can be caused by hypnozoites in the hepatocyte. Since most malarial medications, including chloroquine, cannot kill hypnozoites, primaquine is administered to prevent future recurrences of vivax malaria. The adequate dosage of primaquine differs depending on outbreak regions and reports [3-6].
Although a few studies targeting civilians in the Republic of Korea have been conducted since the re-emergence of malaria, most of the studies analyzed a small group of patients [7, 8]. This study is aimed at analyzing clinical features of vivax malaria and characteristics of recurred malarial patients who were treated at a hospital located in the northwestern part of Gyeonggi province where malaria is prevalent.
This study was conducted in a university-affiliated hospital located in Goyang of Gyeonggi province. Patients over 15 years old diagnosed as vivax malaria by peripheral blood smear between January 2005 and December 2009 were enrolled. We retrospectively reviewed the medical records of patients and collected information including sex, age, weight, hospitalization history, residence, date of symptom onset and diagnosis, fever pattern, previous history of malaria, whether or not patients had participated in military service, underlying diseases, results of blood test and radiology, recurrence, occurrence of complications, and administered doses and period of chloroquine and primaquine. For the recurrent cases, only the first diagnosis was included for the analysis of clinical characteristics. However, all episodes were counted for analysis of monthly and yearly distribution of vivax malaria regardless of recurrence.
Statistical analysis was processed using SAS Enterprise Guide Version 4.1 (SAS Institute Inc., Cary, North Carolina, USA). The normality of the samples was confirmed by performing a Shapiro-Wilk test. Comparisons of clinical characteristics between the first and recurrent episodes of vivax malaria in recurrent patients were performed by paired t-test for samples normally distributed and Wilcoxon signed rank test for samples not normally distributed. For all statistical analyses a P-value less than 0.05 (two-sided) was considered statistically significant.
During the 5-year study period, a total of 352 malarial cases were diagnosed. Vivax malaria most commonly developed in July and August, 87 (24.7%) cases and 77 (21.9%), respectively, followed by 62 (17.6%) cases in September and 54 (15.4%) cases in June (Fig. 1). A total of 334 malarial cases were diagnosed from May to October, accounting for 94.9%, and 2 and 4 cases were detected respectively in December and January. Analysis of yearly distribution showed that cases diagnosed in 2006 and 2007 were most frequent as 90 and 96 cases, respectively.
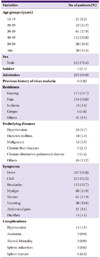
Among 352 malarial cases diagnosed during the study period, 11 cases were recurrent cases developed in same patients. Therefore, a total of 341 patients were included for the analysis. The mean (SD) age of patients was 42.5 (14.7) years and there were 112 patients aged 40-49, making this age group most prevalent with vivax malaria (Table 1). Men numbered 243 (71.3%) and civilians 334 (97.9%), accounting for the biggest proportion of the subjects. Four patients were soldiers actively serving, 3 patients were discharged from military within 2 months of the diagnosis, and 3 out of 5 patients related to military whose medication history was available for review took preventive medication. A total of 225 patients (66.0%) were hospitalized. Residences of the patients were as follows: 173 (50.7%) in Goyang; 118 (34.6%) in Paju; 13 (3.8%) in Incheon; and 6 (1.8%) in Gimpo. Six patients (1.8%) had had a history of malaria before diagnosed at our hospital, and of them 2 patients were suspected of reinfection as it had been more than 5 years since the previous malaria diagnosis. Four patients experienced recurrent episodes of malaria during the study period in 6-13 months after previous treatment. It was not clear whether these patients with a previous history of malaria, except the one patient who had been treated in our hospital, took primaquine at the time. A total of 92 (27.0%) patients had an underlying disease; of them, 33 (9.7%) patients had hypertension which was found to be the most common accompanying disease, and 4 patients were diagnosed while being pregnant.
The mean (SD) time taken to be diagnosed and treated with malaria from the time of first onset of symptoms was 6.5 (3.9) days. A total of 337 (98.8%) patients had an accompanying fever, and the mean (SD) body temperature was 38.3 (1.4)℃. Chills were present in 213 (62.5%) patients, accompanying headache in 115 patients (33.7%), and myalgia in 85 (24.9%) patients. Nausea and vomiting were present respectively in 61 (17.9%) and 36 (10.6%) patients, and 31 (9.1%) patients complained of abdominal pain, with accompanying diarrhea in 14 (4.1%) patients. Regarding the presence of complications, hypotension was present in 14 (4.1%) patients, accompanying azotemia in 3 (0.9%) patients, and of them 3 patients had altered consciousness. Splenic infraction and splenic rupture were diagnosed respectively in 2 and 1 patients, and there were no cases of death caused by malaria.
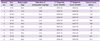
Thrombocytopenia was the sign most commonly identified among test subjects, present in 340 patients (99.7%), and platelet counts were < 100,000/mm3 in 303 (88.9%) patients, < 50,000/mm3 in 148 (43.4%) patients, and severe thrombocytopenia, which is < 20,000/mm3, occurred in 20 (5.9%) patients (Table 2). Other hematological abnormalities were also present; anemia (hemoglobin <12 g/dL) in 97 (28.5%) patients, leukopenia in 148 (43.4%) patients, and leukocytosis in 10 (2.9%) patients. The levels of aspartate transaminase (AST) and alanine transaminase (ALT) were elevated in 177 (51.9%) and 187 (54.8%) patients, respectively. The level of total bilirubin was elevated (>1.5 mg/dL) in 183 (53.7%) patients.
Within a month of diagnosis, 39 patients were examined with abdominal computed tomography and 41 patients with ultrasonography. On radiologic evaluation, hepatomegaly was present in 23 (6.7%) patients and splenomegaly was present in 45 (13.2%) patients.
All patients were treated with either 2,500 mg of chloroquine phosphate (Malachlo, Shin Poong Pharm. Co., Ltd) or 2,000 mg of hydroxchloroquine equivalent to 1,500 mg of chloroquine base within 48 hours. A total of 320 patients received primaquine (Vivaquine, Myung In Pharm. Co. Ltd) except 4 pregnant women and 17 additional patients who did not follow up after diagnosis or whose history of primaquine was uncertain due to being transferred to other hospitals. Primaquine was prescribed for 2 weeks with 15 mg to 280 (87.5%) patients, 22.5 mg to 24 (7.5%), and 30 mg to 8 (2.5%) patients. Eight (7.5%) patients were prescribed less than 10 days and advised to visit the hospital again, however, they did not follow up. The mean (SD) weight-adjusted primaquine dose was 3.39 (0.82) mg/kg.
During the study period, 11 (3.2%) patients developed recurrent episodes. The characteristics of the recurrent patients are described in Table 3. Of the 11 recurrent patients, 10 (90.9%) were men, and the mean (SD) age was 44.8 (11.8) years. There were no soldiers on active duty or discharged within a year, and 7 and 4 patients were residents of Goyang and Paju, respectively. Except for 3 patients who had hypertension, no other underlying diseases were found in the patients. Also, there were no cases in which severe complications occurred, such as severe anemia, shock, renal failure, spleen rupture, spleen infraction, and altered mentality. The mean (SD) weight was 71.7 (12.1) kg. The annual number of recurrent cases was 3 in 2006, 6 in 2007, and 2 in 2009, and all recurrent cases occurred between May and October. The median (range) time taken for recurrent episodes to manifest was 100 (32-385) days; there were no recurrent episodes within 1 month, but there was 1 (9.1%) patient who had a recurrent episode within 5 weeks, 4 (36.4%) patients within 3 months, and 6 (54.5%) patients after 3 months. In the first episode, all 11 patients received chloroquine followed by primaquine 15 mg for 2 weeks. The mean (SD) dose of total primaquine administered when weight-adjusted was 3.01 (0.56) mg/kg, which was relatively less than 3.39 (0.82) mg/kg administered to the patients who did not experience recurrence, yet it was not statistically significant (P=0.057). In the recurrent episode, 15-30 mg of primaquine were administered for 14 to 28 days to the patients, the dose of which was significantly higher compared to that administered in the first episode (P=0.012). In the comparison of blood tests between the first and recurrent episodes, the platelet count in the recurrent episode was significantly higher than in the first episode (P=0.010) (Table 4). In addition, the time taken to administer medication for vivax malaria from the onset of symptoms was significantly shorter in the recurrent episode (P=0.046).
Whereas the average incubation period of vivax malaria that occurs in tropical areas is about 2 weeks, the incubation period of vivax malaria in the Republic of Korea is known to vary from as short as 2 weeks to as long as 6-12 months [1, 9]. Most studies define May through October as the period of high risk for the occurrence of vivax malaria, with a peak in July and August [10-12]. Cases are seen even in late fall through late spring when mosquito vectors are not active, and it is assumed that such cases are attributed to a long incubation period since infection in the previous year [9-10]. In our study, most cases (94.9%, 334/352) were found between May and October with a peak in July and August, and cases were sporadically seen in late fall through late spring.
Vivax malaria seldom causes severe complications or death; however, recurrent episodes originating from hypnozoites of Plasmodium vivax occur after a certain amount of time even though parasites disappeared completely in blood. Therefore, administering chloroquine for 3 days and 15 or 30 mg of primaquine which works on hypnozoites, for 2 weeks is recommended as a standard treatment to prevent relapses of vivax malaria [1, 4, 13]. In our study, the rate of recurrence was 3.2%. Possible causes of recurrent episodes include resistance to chloroquine, recrudescence, resistance to primaquine, inadequate primaquine dose, low compliance with medication, and reinfection [14]. In the Republic of Korea, it is extremely difficult to make a distinction between recrudescence, relapse, and reinfection, since reinfection is always possible during malaria season. Additionally, there is no method to distinguish what, among recrudescence, relapse, and reinfection, causes recurrent episodes [15]. However, the time between the initial episode and a recurrent episode can be a clue to differentiate causes of recurrent episodes. In general, recrudescence, caused by failing to block the intra-erythrocyte life cycle of P. vivax, occurs within 4-5 weeks of treatment whereas relapse caused by extra-erythrocyte life cycle takes longer to occur than recrudescence [9]. If recurrence occurs after a period of 2-3 months, it is more likely to be related to primaquine administration rather than chloroquine resistance [14]. In this study, one patient after 32 days, 4 patients within 3 months, and 6 patients after 3 months of treatment respectively experienced recurrences, and all of those episodes were detected during malaria season, between May and October. Therefore, the possibility of reinfection cannot be ruled out. Chloroquine resistance has not been known to be a serious issue yet in the Republic of Korea; however, military cases that did not respond to a prophylactic dose of chloroquine have been found since 2000 and there were a few reports about chloroquine resistance to the treatment dosage [10, 16]. Moreover, some cases of early recurrence were detected in our study, and therefore, continuous monitoring of the changes in susceptibility to chloroquine is needed.
Inappropriate dosages and compliance of primaquine is reported as one of risk factors for recurrence of vivax malaria, and there are several reports to propose that the dosage of primaquine should be adjusted according to weight [4, 17]. Baird et al. reported that 26 out of 103 patients experienced recurrence when 15 mg of primaquine was administered for 14 days, whereas only 1 out of 36 experienced recurrence when 22.5 or 30 mg was administered for 14 days. Also, they recommended that a dosage of more than 0.5 mg/kg of primaquine should be administered to those who weigh more than 70 kg [4]. Schwartz et al. reported that administering subtherapeutic dose of primaquine to the obese was a cause of primaquine treatment failure, but recurrence did not occur when more than 3.5 mg/kg dose was administered. In addition, Takechi et al. reported that the rate of recurrence was high when the primaquine dose is less than 2.75 mg/kg [17, 18]. Besides age and sex, additionally in the cases where parasitemia is high at the time of diagnosis, the time elapsed from the onset of symptoms to the hospital visit is short, and that gametocyte exists at the time of hospitalization have been reported as factors related to recurrence [18-19]. Since primaquine needs to be administered for 14 days and the main symptoms of malaria, including fever, improve during this time, medication adherence can be expected to play a critical role in preventing recurrence. Takechi et al. reported that a group treated with directly-observed therapy (DOT) had lower rates of re-appearance of P. vivax by comparing with self-administration group ; 15% of the self-administration patients failed to take the medication more than once [18].
In this study, decreased platelet counts, which commonly accompanies vivax malaria, was present more significantly in the first episode in comparison with the recurrent episode (P=0.010), and the time elapsed from the onset of symptoms to treatment as malaria was significantly shorter in the recurrent episode (P=0.046). It is thought that the recurrent patients tend to visit the hospital earlier due to their previous experiences and learning from them, and malaria was also diagnosed more easily by doctors because of the patients' previous histories. Our study was not able to analyze risk factors that cause recurrence since it was not a prospective study and the number of recurrent patients was too small. However, 6 (54.6%) out of 11 recurrent patients weighed over 70 kg, and the primaquine dosage (3.01 mg/kg) administered to recurrent patients tended to be less than that of the non-recurrent group (3.39 mg/kg) even though it is not statistically significant (P=0.057). Therefore, prospective studies on larger numbers of patients examining the influence of primaquine dosage for recurrence in the Republic of Korea are needed.
Our study was conducted with the largest number of recurred patients since the reappearance of vivax malaria in the Republic of Korea. Nevertheless, there are some limitations of this study. This study was not performed as a cohort study, targeting only patients who had visited the hospital, and therefore there is a possibility that those who had visited other hospitals or public health centers for recurrent episode were excluded. In addition, considering the fact that some patients were not included in the analysis of recurrent patients even though they had had a history of vivax malaria, this study may underestimate the rate of recurrence. Although risk factors attributed to recurrence were not analyzed due to the small number of patients and the low rate of recurrence, large prospective studies should be considered to evaluate risk factors related to recurrence of vivax malaria and determine appropriate dosages of primaquine.
Figures and Tables
References
1. Yeom JS. Diagnosis and treatment of vivax malaria. Korean J Med. 2009. 77:52–54.
2. Korea Centers for Disease Control and Prevention. Diseases web statistics system. Accessed on 3 July 2012. Available at: http://stat.cdc.go.kr/.
3. Krudsood S, Tangpukdee N, Wilairatana P, Phophak N, Baird JK, Brittenham GM, Looareesuwan S. High-dose primaquine regimens against relapse of Plasmodium vivax malaria. Am J Trop Med Hyg. 2008. 78:736–740.

5. Goller JL, Jolley D, Ringwald P, Biggs BA. Regional differences in the response of Plasmodium vivax malaria to primaquine as anti-relapse therapy. Am J Trop Med Hyg. 2007. 76:203–207.

6. Pukrittayakamee S, Imwong M, Chotivanich K, Singhasivanon P, Day NP, White NJ. A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg. 2010. 82:542–547.

7. Koo KB, Cho NH, Kim SH, Won YJ, Cho HS. The clinical analysis of 79 cases of indigenous malaia in Myongji hospital during 4 years. J Korean Acad Fam Med. 2004. 25:403–410.
8. Song HH, O SO, Kim SH, Moon SH, Kim JB, Yoon JW, Koo JR, Hong KS, Lee MG, Kim DJ, Shin DH, Kang SH, Choi MG, Lee KH. Clinical features of Plasmodium vivax malaria. Korean J Med. 2002. 63:546–551.

9. Ko DH, Kim SS, Choi BS, Seog W, Kim CH, Cho YK, So BJ, Kim CS. Studies on the vivax malaria readmitted in military hospital. Korean J Med. 2005. 68:611–618.
10. Yeom JS, Park JW. Status of vivax malaria after re-emergence in South Korea. Infect Chemother. 2008. 40:191–198.

11. Park JW. Status of Plasmodium vivax malaria in the Republic of Korea after reemergence. Hanyang Med Rev. 2010. 30:176–186.

13. Centers for Disease Control and Prevention. Treatment of Malaria: Guidelines For Clinicians (United States). Accessed on 3 July 2012. Available at: http://www.cdc.gov/malaria/diagnosis_treatment/clinicians2.html.
14. Yi KJ, Chung MH, Kim HS, Kim CS, Pai SH. A relapsed case of imported tertian malaria after a standard course of hydroxychloroquine and primaquine therapy. Korean J Parasitol. 1998. 36:143–146.

15. Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004. 48:4075–4083.
16. Lee KS, Kim TH, Kim ES, Lim HS, Yeom JS, Jun G, Park JW. Short report: chloroquine-resistant Plasmodium vivax in the Republic of Korea. Am J Trop Med Hyg. 2009. 80:215–217.
17. Schwartz E, Regev-Yochay G, Kurnik D. Short report: a consideration of primaquine dose adjustment for radical cure of Plasmodium vivax malaria. Am J Trop Med Hyg. 2000. 62:393–395.

18. Takeuchi R, Lawpoolsri S, Imwong M, Kobayashi J, Kaewkungwal J, Pukrittayakamee S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, Day NP, Singhasivanon P. Directly-observed therapy (DOT) for the radical 14-day primaquine treatment of Plasmodium vivax malaria on the Thai-Myanmar border. Malar J. 2010. 9:308.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download