Abstract
Antimicrobial resistance has become one of the most serious public health concerns worldwide. Although circumstances may vary by region or country, it is clear that some Asian countries are epicenters of resistance, having seen rapid increases in the prevalence of antimicrobial resistance of major bacterial pathogens. In these locations, however, the public health infrastructure to combat this problem is very poor. The prevalence rates of methicillin-resistant Staphylococcus aureus (MRSA), macrolide-resistant Streptococcus pneumoniae, and multidrug-resistant enteric pathogens are very high due to the recent emergence of extremely drug-resistant gram-negative bacilli in Asia. Because antimicrobial options for these pathogens are extremely limited, infections caused by antimicrobial-resistant bacteria are often associated with inappropriate antimicrobial therapy and poor clinical outcomes. Physicians should be aware of the current epidemiological status of resistance and understand the appropriate use of antimicrobial agents in clinical practice. This review focuses on describing the epidemiology and clinical implications of antimicrobial-resistant bacterial infections in Asian countries.
Antimicrobial resistance has become one of the most serious public health concerns worldwide. It is a global rather than a local issue, as antimicrobial resistance can spread between countries or continents. Massive increases in trade and long-distance travel have enabled the rapid spread of resistant pathogens. The spread of New Delhi metallo-beta-lactamase-1 (NDM-1)-an enzyme that makes bacteria such as Escherichia coli resistant to antibiotics-from India to many Western countries is one of the best recent examples of the transmission of antimicrobial resistance between countries, as it showed the critical impact of antimicrobial resistance on not just public health but also economies and trade [1]. Among community pathogens, penicillin- or macrolide-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA), and multidrug-resistant (MDR) enteric pathogens are of major concern in the Asian region. Among nosocomial pathogens, MRSA or glycopeptide-resistant S. aureus (vancomycin-intermediate or resistant S. aureus, VISA or VRSA, respectively), glycopeptide-resistant enterococci (vancomycin-resistant enterococci, VRE), extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, and MDR nonfermenters are being found with increasing frequency around the world.
Although the circumstances of antimicrobial resistance may vary by region or country, it is clear that Asia is an epicenter of antimicrobial resistance; the prevalence of antimicrobial resistance of major bacterial pathogens has been rapidly increasing. However, public health infrastructures to control the problem are very poor in many countries [2, 3]. Given that more than 70% of the worldwide population lives in the Asia-Pacific region, antimicrobial resistance in this region is, literally, a global problem.
According to published reports, very high prevalence rates of beta-lactam and macrolide resistance in S. pneumoniae have been found in Asian countries [4, 5]. Particularly, erythromycin resistance has increased dramatically in many Asian countries, where > 70% of clinical isolates were fully resistant [4, 5]. According to a recent prospective surveillance study performed by the Asian Network for Surveillance of Resistant Pathogens (ANSORP), the prevalence rates of penicillin resistance were 0.7% and 57.5% in nonmeningeal and meningeal isolates, respectively, based on the revised CLSI breakpoints for parenteral penicillin (resistant ≥ 8 µg/ml for nonmeningeal isolates and ≥ 0.12 µg/ml for meningeal isolates) [4]. Compared with previous ANSORP studies, recent data showed a persistently high prevalence of penicillin nonsusceptibility in Asian countries when previous penicillin susceptibility breakpoints were applied [4-6]. However, according to the revised CLSI breakpoints, the prevalence rate of penicillin-nonsusceptible pneumococci (PNSP) in nonmeningeal isolates was only 4.6%, and fully resistant isolates were found only in China (2.2%) and South Korea (0.3%). Resistance to erythromycin was very prevalent in the region (72.7%); the highest rates were in China (96.4%), Taiwan (84.9%), and Vietnam (80.7%). Multidrug resistance (MDR) was observed in 59.3% of the isolates from Asian countries.
The first report regarding the emergence of fluoroquinolone resistance among S. pneumoniae isolates in Asia was from Hong Kong [7], and a subsequent case-control study showed that the presence of chronic obstructive pulmonary disease, nosocomial origin of the bacteria, residence in a nursing home, and exposure to fluoroquinolones were independently associated with levofloxacin-resistant S. pneumoniae colonization or infection [8]. In a Taiwanese hospital, rates of levofloxacin nonsusceptibility of S. pneumoniae increased significantly from 1.2% in 2001 to 4.2% in 2007 [9]. The ANSORP study showed that the resistance rates to fluoroquinolones were 1.7%, 0.4%, 1.5%, and 13.4% for levofloxacin, moxifloxacin, gatifloxacin, and ciprofloxacin, respectively, in Asian countries [4]. Isolates from Taiwan (6.5%) and South Korea (4.6%) showed the highest rates of levofloxacin resistance [4]. A case of bacteremic pneumonia caused by an extremely drug-resistant strain of S. pneumoniae, nonsusceptible to at least one agent in all classes but vancomycin and linezolid, was reported in Korea [10].
Despite the widespread emergence of in vitro resistance in pneumococcal isolates in Asia, the ANSORP studies showed that mortality rates among patients with pneumococcal pneumonia caused by penicillin-, cephalosporin-, or macrolide-resistant strains were not higher than those among patients with antibiotic-susceptible pneumococcal pneumonia [11, 12]. Even though several studies suggested that the outcome of serious pneumococcal infections was not significantly affected by antimicrobial resistance [12, 13], a post-hoc analysis of the ANSORP study showed that levofloxacin resistance was associated with increased mortality in adult patients with invasive pneumococcal diseases [14]. When the 2008 CLSI penicillin breakpoints were applied, a Korean hospital found that fatal outcomes in patients with nonmeningeal pneumococcal bacteremia attributable to penicillin nonsusceptibility were likely to be rare [15]. However, ceftriaxone nonsusceptibility was found to be one of the independent risk factors for 30-day mortality in the study [15].
Asian countries have shown very high rates (> 50%) of MRSA, which is the most important cause of hospital-acquired infections such as pneumonia, surgical site infections (SSI), and bloodstream infections (Fig. 1). MRSA kills more than 19,000 patients annually in the U.S. alone; thus, many Asian countries could have a huge number of deaths due to this infection [2, 16]. The ANSORP study on S. aureus infections in Asia showed that MRSA accounted for 25.5% of community-associated (CA) S. aureus infections and 67.4% of healthcare-associated (HA) infections [4]. The proportion of MRSA among HA S. aureus infections was relatively low in India (22.6%) and the Philippines (38.1%), whereas Sri Lanka (86.5%), Korea (77.6%), and Vietnam (74.1%) showed very high rates of MRSA in the ANSORP study. Hospital acquired pneumonia (HAP) caused by S. aureus in Asian countries had high resistance rates to oxacillin (82.1%), ciprofloxacin (78.2%), clindamycin (64.2%), erythromycin (76.5%), and tetracycline (70.9%)[17]. Among all S. aureus isolates causing ventilator-associated pneumonia in Chinese hospitals, 45.7% were methicillin-resistant [18].
In recent years, community-associated MRSA (CA-MRSA) infections have emerged worldwide [19, 20]. The ANSORP study showed that the proportion of MRSA in CA S. aureus infections varied by country: Sri Lanka (38.8%); Taiwan (34.8%); the Philippines (30.1%); Vietnam (30.1%); Korea (15.6%); Hong Kong (8.5%); India (4.3%); and Thailand (2.5%) [21]. In a study on CA-MRSA among patients with purulent skin and soft tissue infections in Hong Kong, 10.4% (13/125) of all S. aureus isolates and 5% (12/241) of all abscesses were attributed to panton-valetine leukocidin (PVL)-positive CA-MRSA [22]. A prospective cohort study on MRSA carriage conducted over 1 month at a children's hospital in Cambodia identified MRSA carriage in 87 (3.5%) of 2,485 children who came to the outpatient department and in 6 (4.1%) of 145 inpatients [23]. In a Taiwanese hospital, the number of adult patients with CA-MRSA bacteremia increased over time, and the disease was associated with more cases of necrotizing pneumonia and cutaneous abscess but fewer cases of endovascular infection compared with CA-MSSA bacteremia [24]. Patients with CA-MRSA bacteremia did not experience higher mortality than patients with CA-MSSA, even though most of the former did not receive empirical glycopeptide therapy [24].
A systematic review and meta-analysis showed that the mortality rate of MRSA bacteremia was significantly higher than that of MSSA bacteremia [25]. The ANSORP study, which included 4,949 patients with S. aureus infection in Asian countries, showed that methicillin resistance adversely affected the outcomes of patients with S. aureus infection, especially in patients with cancer or renal disease, and in those with S. aureus bacteremia, although MRSA infection was not found to be significantly associated with higher mortality in the overall patient population [26]. In a study on S. aureus bacteremia in northern Thailand, a higher mortality rate (52%) than that in industrialized countries was reported, and mortality rates for MRSA and MSSA were 67% and 46%, respectively (P=0.11) [27].
Although the prevalence of VISA or VRSA infections is low in Asia [21], a significant problem associated with MRSA is the subpopulation of MRSA with reduced vancomycin susceptibility (also known as heterogeneous vancomycin-intermediate-resistant S. aureus, hVISA), particularly in Japan [2, 28]. However, multinational surveillance studies in Asia have found that the prevalence of MRSA with reduced vancomycin susceptibility ranges from 0.7% to 4.3% [29, 30]. In the ANSORP study on HAP in Asian countries, VISA or VRSA was not found [17]. Clonal dissemination of VISA has been reported in a Taiwanese hospital [31]. The hVISA phenotype was present in more than one-third of MRSA isolates in a Korean hospital [32] and was independently associated with a vancomycin MIC ≥2 mg/L, rifampicin resistance, prior vancomycin therapy, and use of immunosuppressive therapy. Compared with vancomycin-susceptible S. aureus, hVISA and VISA infections were found to be associated with a longer period of prior glycopeptide use, bone/joint and prosthetic infections, and treatment failure, as evidenced by the longer bacteremic and culture positivity periods in a Singapore hospital [33]. There was, however, no significant difference in overall patient mortality or hospitalization costs between the two groups [33].
The prevalence of VRE, which emerged in the late 1980s, has risen rapidly in many countries. Its prevalence among clinical isolates has been estimated to range from 12% to 21% in Korea, and similar estimates have been made in Taiwan [2, 34, 35]. The prevalence of non-duplicated blood VRE isolates in a Taiwanese hospital increased significantly from 3.9% in 2003 to 18.9% in 2010 [36]. In Chinese hospitals, the prevalence of VRE increased from 0 in 2005 to 4.9% in 2010, and among the VRE isolates, the vanA gene was the most prevalent gene [37]. Among patients admitted to the ICU in a Korean hospital, 3.4% carried VRE; independent risk factors for VRE carriage at ICU admission were ICU readmission during hospitalization, chronic obstructive lung disease, recent antibiotic treatment, and recent vancomycin use [38]. In addition, the increment of VRE colonization pressure was significantly associated with vancomycin consumption 1 week before and moderately associated with that of the corresponding week [39].
A systematic review and meta-analysis showed that vancomycin resistance was independently associated with increased mortality among patients with enterococcal bloodstream infections [40]. Additionally, enterococcal bacteremia was associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation [41]. On the other hand, among patients with VRE bacteremia in a Korean hospital, mortality did not differ between those receiving anti-VRE therapy later than 72 h after the onset of bacteremia and those receiving treatment within 72 h; these findings suggest that, despite antibiotic therapy to fight VRE, patients with VRE bacteremia eventually have a higher risk of death because of severe illness at the onset of bacteremia [42].
The Study for Monitoring Antimicrobial Resistance Trends (SMART) was initiated in 2002 to longitudinally monitor the in vitro susceptibility profiles of aerobic and facultative gram-negative bacilli isolated from patients with intra-abdominal infections, and it was expanded to include surveillance of gram-negative pathogens causing urinary tract infections in the Asia-Pacific region [43, 44]. Data from previous SMART studies have revealed that the levels of antimicrobial resistance are highest in countries in the Asia-Pacific region. Among gram-negative bacilli collected from intra-abdominal infections in the Asia-Pacific region during 2007, 42.2% and 35.8% of E. coli and Klebsiella spp., respectively, were ESBL positive [45]. Moreover, the ESBL rates in India for E. coli, K. pneumoniae, and K. oxytoca were 79.0%, 69.4%, and 100%, respectively [45]. Among Enterobacteriaceae isolates collected from 8 tertiary-care hospitals in various regions of Korea, ESBL positivity of K. pneumoniae isolates was 22.4%, while that of E. coli isolates was 10.2% [46]. The prevalences of Enterobacteriaceae isolates with ESBL were 26% in K. pneumoniae, 14% in E. coli, and 13% in Proteus mirabilis, and a significantly rising prevalence of ESBL production among K. pneumoniae was noted in Taiwanese intensive care units [47]. In a Korean hospital, antibiotic resistance in bacteremic biliary tract infections has increased markedly over the past 10 years, and almost half of the nosocomial bacteremic biliary tract infections caused by common gram-negative pathogens during 2005-2009 could not be treated with third-generation cephalosporins [48]. Figure 2 shows the prevalence of ESBL-producers among E. coli and K. pneumoniae isolates causing urinary tract infections in the Asia-Pacific region [44].
ESBL-producing E. coli are increasingly prevalent pathogens in community and healthcare settings. The international dissemination of blaCTX-M ESBL genes and ST131 over the last decade has been described as a pandemic. CTX-M-producing E. coli and ST131 have emerged as a significant cause of both community-onset and hospital-acquired infections in Asian countries, including Korea [49], Taiwan [50], China [51], Hong Kong [52], Japan [53], Malaysia [54], and Thailand [55], and the incidence of serious infections due to CTX-M-producing E. coli likely will continue to increase. CTX-M-producing E. coli have been highly endemic worldwide. In a Taiwanese hospital, patients with clone ST131 were more likely to have secondary bacteremia and noncatheterized urinary tract infections [50]. Although more virulence factors have been detected in ST131, patients with the ST131 clone in ESBL-producing E. coli bacteremia did not exhibit a higher mortality rate [50].
A systematic review and meta-analysis showed that ESBL production is associated with increased mortality and a delay in effective therapy in Enterobacteriaceae bacteremia [56]. In a study in Korea, ESBL-producing bacteremia was the most important risk factor associated with 30-day mortality in patients with hematologic malignancy, along with ICU care and a higher Pitt bacteremia score [57], although the difference in the mortality rates for ESBL bacteremia and non-ESBL bacteremia was not significant in another study [58]. A Korean nationwide study on community-onset bacteremia caused by ESBL-producing E. coli showed that ESBL production was an independent factor associated with mortality after adjusting for confounding variables, suggesting that ESBL-producing E. coli is a significant cause of bacteremia even in patients with community-onset infections [59]. However, in other studies on community-onset bacteremia caused by ESBL-producing E. coli or K. pneumoniae, increased mortality was not statistically associated with either ESBL production or inappropriate empirical therapy [60, 61]. Similarly, in a Chinese study regarding community-acquired intra-abdominal infections caused by ESBL-producing bacteremia, patients with ESBL-positive infections had similar resolution rates at discharge compared to those with ESBL-negative infection, despite poorer first-line antibiotic responses [62]. However, ESBL-positive infections led to significantly higher costs from hospitalization and intravenous antibiotics, as well as longer hospital stays [62].
Carbapenem-resistant Enterobacteriaceae (CRE) are particularly problematic, given the frequency with which Enterobacteriaceae cause infections and the potential for widespread transmission of carbapenem resistance via mobile genetic elements [63]. The most important carbapenemases among Enterobacteriaceae clinical isolates are KPC and NDM. While KPC-producing organisms are rarely reported in Asian countries [64, 65], NDM-producing organisms are prevalent among Enterobacteriaceae in India and Pakistan, even in community-onset infections [1]. NDM-1 carbapenemase-producing Enterobacteriaceae strains have been detected worldwide [2].
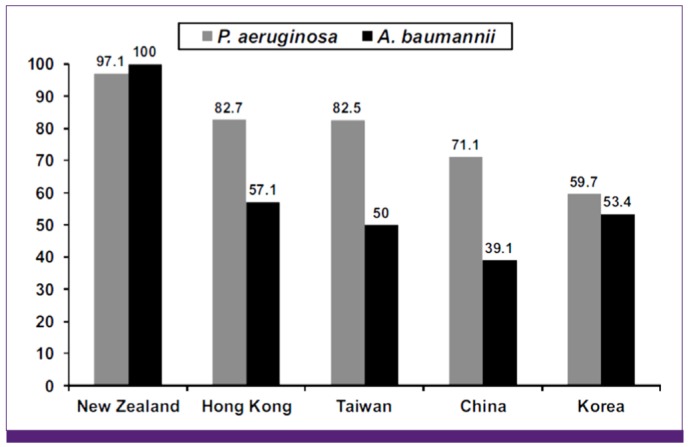
The rate of carbapenem-resistant Pseudomonas aeruginosa is very high, and MDR nonfermenters are highly prevalent in Asian countries [34, 35]. In the ANSORP study on HAP, resistance rates of P. aeruginosa to ceftazidime, cefepime, piperacillin-tazobactam, imipenem, and ciprofloxacin were 34.7%, 27.7%, 36.9%, 27.2%, and 30.1%, respectively [17]. The resistance rate of P. aeruginosa to imipenem was the highest in China (56.9%). HAP associated with Acinetobacter spp. in Asian countries showed a very high rate of resistance to imipenem, at 67.3%; the rate was especially high in Malaysia (86.7%), Thailand (81.4%), India (85.7%), and China (58.9%) [17]. MDR and XDR rates of Acinetobacter spp. were 82.0% and 51.1%, respectively. The prevalence of imipenem-resistant A. baumannii has been rising gradually in Korea, as well as in China and Taiwan [34, 66-68]. Rates of carbapenem-resistant Acinetobacter spp. and P. aeruginosa were very high, and MDR nonfermenters were highly prevalent in Asian countries [17, 34, 35]. In a surveillance study in the Asia-Pacific region, 29.8% of P. aeruginosa and 73.0% of A. baumannii isolates were not susceptible to at least one carbapenem, whereas the majority of Enterobacteriaceae (97.2%) were susceptible to all carbapenems [69]. The SMART study on intra-abdominal infections in the Asia-Pacific region showed that A. baumannii exhibited very high rates of resistance to most antimicrobial agents, including imipenem [70]. Figure 3 shows the results of antimicrobial susceptibility to imipenem among isolates of P. aeruginosa and A. baumannii obtained from patients with intra-abdominal infections in selected countries in the Asia-Pacific region [70].
Despite the high prevalence of MDR or XDR Pseudomonas and Acinetobacter in Asia [2, 68], the clinical consequences of antimicrobial resistance are not fully understood in many Asian countries. In a Korean hospital, antimicrobial resistance, especially to ceftazidime and imipenem, adversely affected the outcomes of patients with P. aeruginosa bacteremia [71]. In a multicenter study in Taiwan, patients with carbapenem-resistant A. baumannii bacteremia had a higher mortality rate than patients with carbapenem-susceptible A. baumannii bacteremia (46.0% vs. 28.3%, P=0.04), and multivariate analysis showed that carbapenem resistance was one of the independent variables associated with mortality in patients with A. baumannii bacteremia [72]. In a previous study in Korea, imipenem resistance had a significant impact on mortality among patients with Acinetobacter bacteremia, and this was mainly attributable to the higher rate of discordant antimicrobial therapy [73]. The ANSORP study showing a high prevalence of MDR nonfermenters in HAP demonstrated that discordant initial empirical antimicrobial therapy significantly increases the likelihood of pneumonia-related mortality [17].
Asia is one of the epicenters of antimicrobial resistance worldwide, and this is an increasing public health concern. MDR pathogens have been widely disseminated, both in hospitals and throughout communities, in many countries. Continuous surveillance is essential for providing information on the magnitude of, and trends in, antimicrobial resistance. Given the devastating impact of this problem on human lives and public health, future strategies should be developed based on multifaceted collaboration among all relevant stakeholders in the Asia-Pacific region. Comprehensive strategies for the control and prevention of antimicrobial resistance are urgently needed in the region.
Acknowledgments
We would like to thank Young Eun Ha, So Yeon Park, Sun Young Cho, and Jungok Kim for the literature review. This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A102065).
References
1. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010; 10:597–602. PMID: 20705517.

2. Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011; 37:291–295. PMID: 21382699.

3. Nickerson EK, West TE, Day NP, Peacock SJ. Staphylococcus aureus disease and drug resistance in resource-limited countries in south and east Asia. Lancet Infect Dis. 2009; 9:130–135. PMID: 19179228.

4. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, Carlos CC, Pham HV, Lalitha MK, Shimono N, Perera J, Shibl AM, Baek JY, Kang CI, Ko KS, Peck KR. ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–1426. PMID: 22232285.

5. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, Ki HK, Oh WS, Suh JY, Peck KR, Lee NY, Yang Y, Lu Q, Chongthaleong A, Chiu CH, Lalitha MK, Perera J, Yee TT, Kumarasinghe G, Jamal F, Kamarulzaman A, Parasakthi N, Van PH, Carlos C, So T, Ng TK, Shibl A. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–2107. PMID: 15155207.
6. Song JH, Lee NY, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chongthaleong A, Aswapokee N, Chiu CH, Lalitha MK, Thomas K, Perera J, Yee TT, Jamal F, Warsa UC, Vinh BX, Jacobs MR, Appelbaum PC, Pai CH. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin Infect Dis. 1999; 28:1206–1211. PMID: 10451154.
7. Ho PL, Que TL, Tsang DN, Ng TK, Chow KH, Seto WH. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999; 43:1310–1313. PMID: 10223962.
8. Ho PL, Tse WS, Tsang KW, Kwok TK, Ng TK, Cheng VC, Chan RM. Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: a case-control study. Clin Infect Dis. 2001; 32:701–707. PMID: 11229837.

9. Hsieh YC, Chang LY, Huang YC, Lin HC, Huang LM, Hsueh PR. Circulation of international clones of levofloxacin non-susceptible Streptococcus pneumoniae in Taiwan. Clin Microbiol Infect. 2010; 16:973–978. PMID: 19778298.

10. Kang CI, Baek JY, Jeon K, Kim SH, Chung DR, Peck KR, Lee NY, Song JH. Bacteremic pneumonia caused by extensively drug-resistant Streptococcus pneumoniae. J Clin Microbiol. 2012; 50:4175–4177. PMID: 23052301.

11. Kang CI, Song JH, Kim SH, Chung DR, Peck KR, Thamlikitkul V, Wang H, So TM, Hsueh PR, Yasin RM, Carlos CC, Van PH, Perera J. Risk factors and pathogenic significance of bacteremic pneumonia in adult patients with community-acquired pneumococcal pneumonia. J Infect. 2013; 66:34–40. PMID: 22922634.

12. Song JH, Jung SI, Ki HK, Shin MH, Ko KS, Son JS, Chang HH, Kim SW, Lee H, Kim YS, Oh WS, Peck KR, Chongthaleong A, Lalitha MK, Perera J, Yee TT, Jamal F, Kamarulzaman A, Carlos CC, So T. Asian Network for Surveillance of Resistant Pathogens Study Group. Clinical outcomes of pneumococcal pneumonia caused by antibiotic-resistant strains in asian countries: a study by the Asian Network for Surveillance of Resistant Pathogens. Clin Infect Dis. 2004; 38:1570–1578. PMID: 15156445.

13. Song JS, Choe PG, Song KH, Park WB, Park SW, Kim HB, Oh MD, Kim EC, Kim NJ. Risk factors for 30-day mortality in adult patients with pneumococcal bacteraemia, and the impact of antimicrobial resistance on clinical outcomes. Epidemiol Infect. 2012; 140:1267–1276. PMID: 21906414.

14. Kang CI, Song JH, Kim SH, Chung DR, Peck KR, Thamlikitkul V, Wang H, So TM, Hsueh PR, Yasin RM, Carlos CC, Van PH, Perera J. ian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Association of levofloxacin resistance with mortality in adult patients with invasive pneumococcal diseases: a post hoc analysis of a prospective cohort. Infection. 2013; 41:151–157. PMID: 22821428.

15. Choi SH, Chung JW, Sung H, Kim MN, Kim SH, Lee SO, Kim YS, Woo JH, Choi SH. Impact of penicillin nonsusceptibility on clinical outcomes of patients with nonmeningeal Streptococcus pneumoniae bacteremia in the era of the 2008 clinical and laboratory standards institute penicillin breakpoints. Antimicrob Agents Chemother. 2012; 56:4650–4655. PMID: 22687517.

16. Grundmann H, Hellriegel B. Mathematical modelling: a tool for hospital infection control. Lancet Infect Dis. 2006; 6:39–45. PMID: 16377533.

17. Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang CI, Peck KR. Asian Network for Surveillance of Resistant Pathogens Study Group. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011; 184:1409–1417. PMID: 21920919.

18. Xie DS, Xiong W, Lai RP, Liu L, Gan XM, Wang XH, Wang M, Lou YX, Fu XY, Wang HF, Xiang H, Xu YH, Nie SF. Ventilatorassociated pneumonia in intensive care units in Hubei Province, China: a multicentre prospective cohort survey. J Hosp Infect. 2011; 78:284–288. PMID: 21511367.

19. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003; 290:2976–2984. PMID: 14665659.
20. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003; 9:978–984. PMID: 12967497.
21. Song JH, Hsueh PR, Chung DR, Ko KS, Kang CI, Peck KR, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van PH. ANSORP Study Group. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J Antimicrob Chemother. 2011; 66:1061–1069. PMID: 21393157.

22. Ho PL, Chuang SK, Choi YF, Lee RA, Lit AC, Ng TK, Que TL, Shek KC, Tong HK, Tse CW, Tung WK, Yung RW. Hong Kong CA-MRSA surveillance network. Community-associated methicillin-resistant and methicillin-sensitive Staphylococcus aureus: skin and soft tissue infections in Hong Kong. Diagn Microbiol Infect Dis. 2008; 61:245–250. PMID: 18272316.

23. Nickerson EK, Wuthiekanun V, Kumar V, Amornchai P, Wongdeethai N, Chheng K, Chantratita N, Putchhat H, Thaipadungpanit J, Day NP, Peacock SJ. Emergence of community-associated methicillin-resistant Staphylococcus aureus carriage in children in Cambodia. Am J Trop Med Hyg. 2011; 84:313–317. PMID: 21292906.

24. Wang JL, Chen SY, Wang JT, Wu GH, Chiang WC, Hsueh PR, Chen YC, Chang SC. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis. 2008; 46:799–806. PMID: 18266610.

25. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003; 36:53–59. PMID: 12491202.
26. Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, Hsueh PR, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van Pham H. Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect. 2010; 61:299–306. PMID: 20670652.

27. Nickerson EK, Hongsuwan M, Limmathurotsakul D, Wuthiekanun V, Shah KR, Srisomang P, Mahavanakul W, Wacharaprechasgul T, Fowler VG, West TE, Teerawatanasuk N, Becher H, White NJ, Chierakul W, Day NP, Peacock SJ. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One. 2009; 4:e4308. PMID: 19180198.

28. Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997; 350:1670–1673. PMID: 9400512.

29. Ho CM, Hsueh PR, Liu CY, Lee SY, Chiueh TS, Shyr JM, Tsao SM, Chuang YC, Yan JJ, Wang LS, Wang JH, Ho MW, Tien N, Lu JJ. Prevalence and accessory gene regulator (agr) analysis of vancomycin-intermediate Staphylococcus aureus among methicillin-resistant isolates in Taiwan--SMART program, 2003. Eur J Clin Microbiol Infect Dis. 2010; 29:383–389. PMID: 20155296.

30. Song JH, Hiramatsu K, Suh JY, Ko KS, Ito T, Kapi M, Kiem S, Kim YS, Oh WS, Peck KR, Lee NY. Asian Network for Surveillance of Resistant Pathogens Study Group. Emergence in Asian countries of Staphylococcus aureus with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2004; 48:4926–4928. PMID: 15561884.
31. Hsueh PR, Lee SY, Perng CL, Chang TY, Lu JJ. Clonal dissemination of meticillin-resistant and vancomycin-intermediate Staphylococcus aureus in a Taiwanese hospital. Int J Antimicrob Agents. 2010; 36:307–312. PMID: 20685086.

32. Park KH, Kim ES, Kim HS, Park SJ, Bang KM, Park HJ, Park SY, Moon SM, Chong YP, Kim SH, Lee SO, Choi SH, Jeong JY, Kim MN, Woo JH, Kim YS. Comparison of the clinical features, bacterial genotypes and outcomes of patients with bacteraemia due to heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-susceptible S. aureus. J Antimicrob Chemother. 2012; 67:1843–1849. PMID: 22535621.

33. Fong RK, Low J, Koh TH, Kurup A. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur J Clin Microbiol Infect Dis. 2009; 28:983–987. PMID: 19387707.

34. Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, Park YJ, Yong D, Jeong SH, Chong Y. KONSAR Group. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011; 52:793–802. PMID: 21786445.

35. Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S, Kim JJ, Koh E, Yong D, Chong Y. KONSAR Group. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010; 51:901–911. PMID: 20879058.
36. Lu CL, Chuang YC, Chang HC, Chen YC, Wang JT, Chang SC. Microbiological and clinical characteristics of vancomycin-resistant Enterococcus faecium bacteraemia in Taiwan: implication of sequence type for prognosis. J Antimicrob Chemother. 2012; 67:2243–2249. PMID: 22618861.

37. Zhao C, Sun H, Wang H, Liu Y, Hu B, Yu Y, Sun Z, Chu Y, Cao B, Liao K, Lei J, Hu Z, Zhang L, Zhang X, Xu Y, Wang Z, Chen M. Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in China: results from the Gram-Positive Cocci Resistance Surveillance program (2005-2010). Diagn Microbiol Infect Dis. 2012; 73:174–181. PMID: 22521693.

38. Yoon YK, Kim HJ, Lee WJ, Lee SE, Yang KS, Park DW, Sohn JW, Kim MJ. Clinical prediction rule for identifying patients with vancomycin-resistant enterococci (VRE) at the time of admission to the intensive care unit in a low VRE prevalence setting. J Antimicrob Chemother. 2012; 67:2963–2969. PMID: 22888271.

39. Yoon YK, Lee SE, Lee J, Kim HJ, Kim JY, Park DW, Sohn JW, Kim MJ. Risk factors for prolonged carriage of vancomycin-resistant Enterococcus faecium among patients in intensive care units: a case-control study. J Antimicrob Chemother. 2011; 66:1831–1838. PMID: 21652622.

40. DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005; 41:327–333. PMID: 16007529.

41. Vydra J, Shanley RM, George I, Ustun C, Smith AR, Weisdorf DJ, Young JA. Enterococcal bacteremia is associated with increased risk of mortality in recipients of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012; 55:764–770. PMID: 22693346.

42. Han SH, Chin BS, Lee HS, Jeong SJ, Choi HK, Kim CO, Yong D, Choi JY, Song YG, Lee K, Kim JM. Vancomycin-resistant enterococci bacteremia: risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J Infect. 2009; 58:182–190. PMID: 19233476.

43. Chow JW, Satishchandran V, Snyder TA, Harvey CM, Friedland IR, Dinubile MJ. In vitro susceptibilities of aerobic and facultative gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2002 Study for Monitoring Antimicrobial Resistance Trends (SMART). Surg Infect (Larchmt). 2005; 6:439–448. PMID: 16433608.
44. Lu PL, Liu YC, Toh HS, Lee YL, Liu YM, Ho CM, Huang CC, Liu CE, Ko WC, Wang JH, Tang HJ, Yu KW, Chen YS, Chuang YC, Xu Y, Ni Y, Chen YH, Hsueh PR. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents. 2012; 40(Suppl):S37–S43. PMID: 22749057.

45. Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob Agents Chemother. 2009; 53:3280–3284. PMID: 19506060.
46. Ko KS, Lee MY, Song JH, Lee H, Jung DS, Jung SI, Kim SW, Chang HH, Yeom JS, Kim YS, Ki HK, Chung DR, Kwon KT, Peck KR, Lee NY. Prevalence and characterization of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated in Korean hospitals. Diagn Microbiol Infect Dis. 2008; 61:453–459. PMID: 18482815.
47. Jean SS, Hsueh PR, Lee WS, Chang HT, Chou MY, Chen IS, Wang JH, Lin CF, Shyr JM, Ko WC, Wu JJ, Liu YC, Huang WK, Teng LJ, Liu CY. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur J Clin Microbiol Infect Dis. 2009; 28:215–220. PMID: 18716805.

48. Sung YK, Lee JK, Lee KH, Lee KT, Kang CI. The clinical epidemiology and outcomes of bacteremic biliary tract infections caused by antimicrobial-resistant pathogens. Am J Gastroenterol. 2012; 107:473–483. PMID: 22334249.

49. Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, Park C, Choi JH, Yoo JH. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect Dis. 2012; 12:149. PMID: 22747570.

50. Chung HC, Lai CH, Lin JN, Huang CK, Liang SH, Chen WF, Shih YC, Lin HH, Wang JL. Bacteremia caused by extendedspectrum-beta-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob Agents Chemother. 2012; 56:618–622. PMID: 22123694.
51. Cao X, Cavaco LM, Lv Y, Li Y, Zheng B, Wang P, Hasman H, Liu Y, Aarestrup FM. Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J Clin Microbiol. 2011; 49:2496–2501. PMID: 21525216.

52. Ho PL, Lo WU, Lai EL, Chow KH, Yam WC. Escherichia coli O25b-ST131 is an important cause of antimicrobialresistant infections in women with uncomplicated cystitis. J Antimicrob Chemother. 2012; 67:2534–2535. PMID: 22729922.

53. Matsumura Y, Yamamoto M, Nagao M, Hotta G, Matsushima A, Ito Y, Takakura S, Ichiyama S. Kyoto-Shiga Clinical Microbiology Study Group. Emergence and spread of B2-ST131-O25b, B2-ST131-O16 and D-ST405 clonal groups among extended-spectrum-beta-lactamase-producing Escherichia coli in Japan. J Antimicrob Chemother. 2012; 67:2612–2620. PMID: 22843833.
54. Ho WS, Balan G, Puthucheary S, Kong BH, Lim KT, Tan LK, Koh XP, Yeo CC, Thong KL. Prevalence and characterization of multidrug-resistant and extended-spectrum beta-lactamase-producing Escherichia coli from pediatric wards of a Malaysian hospital. Microb Drug Resist. 2012; 18:408–416. PMID: 22394084.
55. Kiratisin P, Apisarnthanarak A, Laesripa C, Saifon P. Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob Agents Chemother. 2008; 52:2818–2824. PMID: 18505851.
56. Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007; 60:913–920. PMID: 17848376.
57. Kang CI, Chung DR, Ko KS, Peck KR, Song JH. Korean Network for Study of Infectious Diseases. Risk factors for infection and treatment outcome of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol. 2012; 91:115–121. PMID: 21556875.
58. Kim SH, Kwon JC, Choi SM, Lee DG, Park SH, Choi JH, Yoo JH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. Escherichia coli and Klebsiella pneumoniae bacteremia in patients with neutropenic fever: factors associated with extended-spectrum β-lactamase production and its impact on outcome. Ann Hematol. 2013; 92:533–541. PMID: 23161391.

59. Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Ki HK, Son JS, Lee SS, Kim YS, Jung SI, Kim SW, Chang HH, Ryu SY, Kwon KT, Lee H, Moon C, Shin SY. Korean Network for Study of Infectious Diseases (KONSID). Risk factors and treatment outcomes of community-onset bacteraemia caused by extended-spectrum beta-lactamase-producing Escherichia coli. Int J Antimicrob Agents. 2010; 36:284–287. PMID: 20580534.
60. Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, Kwon JC, Yoo JH. Emergence of extended-spectrum β-lactamase-producing escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011; 17:537–544. PMID: 21875342.

61. Lee JA, Kang CI, Joo EJ, Ha YE, Kang SJ, Park SY, Chung DR, Peck KR, Ko KS, Lee NY, Song JH. Epidemiology and clinical features of community-onset bacteremia caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Microb Drug Resist. 2011; 17:267–273. PMID: 21388296.

62. Hu B, Ye H, Xu Y, Ni Y, Hu Y, Yu Y, Huang Z, Ma L. Clinical and economic outcomes associated with community-acquired intra-abdominal infections caused by extended spectrum beta-lactamase (ESBL) producing bacteria in China. Curr Med Res Opin. 2010; 26:1443–1449. PMID: 20394469.

63. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011; 53:60–67. PMID: 21653305.

64. Yang Q, Wang H, Sun H, Chen H, Xu Y, Chen M. Phenotypic and genotypic characterization of Enterobacteriaceae with decreased susceptibility to carbapenems: results from large hospital-based surveillance studies in China. Antimicrob Agents Chemother. 2010; 54:573–577. PMID: 19805565.
65. Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009; 9:228–236. PMID: 19324295.

66. Jean SS, Hsueh PR, Lee WS, Chang HT, Chou MY, Chen IS, Wang JH, Lin CF, Shyr JM, Ko WC, Wu JJ, Liu YC, Huang WK, Teng LJ, Liu CY. Nationwide surveillance of antimicrobial resistance among non-fermentative Gram-negative bacteria in Intensive Care Units in Taiwan: SMART programme data 2005. Int J Antimicrob Agents. 2009; 33:266–271. PMID: 19091522.

67. Wang H, Chen M, Ni Y, Liu Y, Sun H, Yu Y, Yu X, Mei Y, Liu M, Sun Z, Chu Y, Hu Z, Huang X. Antimicrobial resistance among clinical isolates from the Chinese Meropenem Surveillance Study (CMSS), 2003-2008. Int J Antimicrob Agents. 2010; 35:227–234. PMID: 20047820.

68. Kuo SC, Chang SC, Wang HY, Lai JF, Chen PC, Shiau YR, Huang IW, Lauderdale TL. TSAR Hospitals. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis. 2012; 12:200. PMID: 22929085.

69. Kiratisin P, Chongthaleong A, Tan TY, Lagamayo E, Roberts S, Garcia J, Davies T. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: results of Asia-Pacific surveillance from the COMPACT II study. Int J Antimicrob Agents. 2012; 39:311–316. PMID: 22386743.

70. Liu YM, Chen YS, Toh HS, Huang CC, Lee YL, Ho CM, Lu PL, Ko WC, Chen YH, Wang JH, Tang HJ, Yu KW, Liu YC, Chuang YC, Xu Y, Ni Y, Liu CE, Hsueh PR. In vitro susceptibilities of non-Enterobacteriaceae isolates from patients with intra-abdominal infections in the Asia-Pacific region from 2003 to 2010: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents. 2012; 40(Suppl):S11–S17. PMID: 22749053.

71. Joo EJ, Kang CI, Ha YE, Kang SJ, Park SY, Chung DR, Peck KR, Lee NY, Song JH. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia: clinical impact of antimicrobial resistance on outcome. Microb Drug Resist. 2011; 17:305–312. PMID: 21381966.

72. Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, Lau YJ, Wang LH, Liu KS, Tsai TY, Lin SY, Hsu MS, Hsu LY, Chang SC. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010; 14:e764–e769. PMID: 20646946.

73. Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, Ryu SY, Heo ST, Jung DS, Rhee JY, Shin SY, Ko KS, Peck KR, Lee NY. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007; 59:525–530. PMID: 17213265.

Figure 1
Prevalence of methicillin resistance among S. aureus isolates.
Some Asian countries have shown the highest prevalence rates of MRSA.
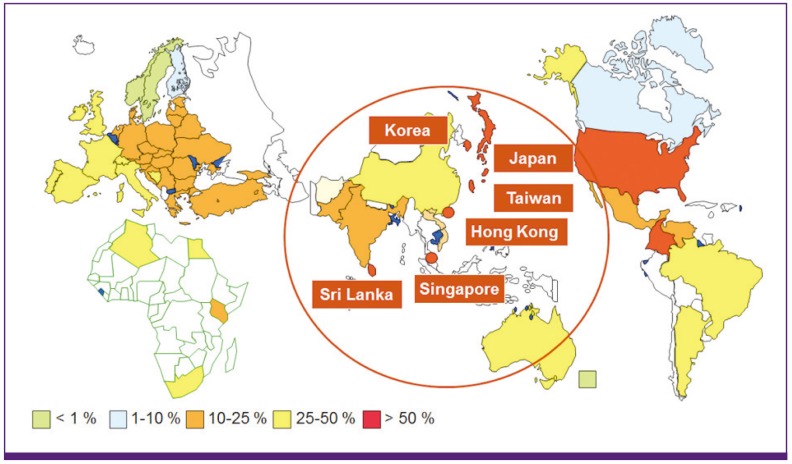
Figure 2
Prevalence of ESBL-producers among E. coli and K. pneumoniae isolates causing urinary tract infections by country in the Asia-Pacific region, adopted from reference [44].
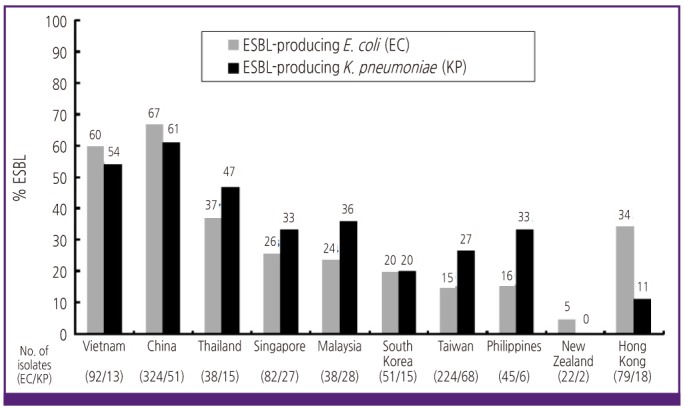
Figure 3
Susceptibility to imipenem among isolates of P. aeruginosa and A. baumannii obtained from patients with intra-abdominal infections in selected countries in the Asia-Pacific region, adopted from reference [70].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download