Abstract
Corynebacterium macginleyi is usually isolated from the eye surfaces and causes ocular infections such as conjunctivitis, keratitis, and endophthalmitis. However, cases that describe C. macginleyi as the causative agent for significant and life-threatening infections in immunocompromised patients are increasingly reported. Herein we report the first documented case of C. macginleyi pneumonia in a human immunodeficiency virus (HIV) patient. A 42-year-old homosexual man with HIV infection was hospitalized with a 1-month history of fever and dry cough. Chest radiograph revealed ill defined ground glass opacities in both lung fields. Methenamine silver stain of bronchoalveolar lavage fluid was negative. He showed clinical improvement after treatment with trimethoprim/sulfamethoxazole and prednisolone for three weeks, and was discharged. One month later, he presented with dyspnea and more progressive pulmonary infiltrations. Bronchial washing fluid culture yielded >100,000 colonies/mL of C. macginleyi, and he was given a 14-day course of antibiotic therapy with vancomycin, after which the patient fully recovered. This case suggest the importance of not overlooking the significance of positive cultures for C. macginleyi obtained from representative clinical samples in patients with signs and symptoms of bacterial infection.
Although Corynebacterium species are ubiquitous gram-positive pleomorphic aerobes that colonize the skin and mucous membranes in humans, they rarely account for clinical infections [1]. Until recently, the pathogenic potential of coryneform bacteria has been underestimated and has been overlooked as a mere skin contamination. However, recent reports show that C. macginleyi isolated from the ocular sites can be the cause of conjunctivitis, keratitis, and endophthalmitis [2-5]. In addition, reports that describe Corynebacterium species as the causative agents for significant and life-threatening infections such as pneumonia, vertebral osteomyelitis, bacteremia, device related infections, endocarditis, and abscesses in immunocompromised patients are increasingly presented [6-8]. Herein we present for the first time the possible involvement of a C. macginleyi strain as the causative agent of pneumonia in a human immunodeficiency virus (HIV) patient.
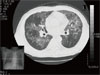
A 42-year-old homosexual man was hospitalized with a 1-month history of fever. He complained of dry cough, weakness, fatigue, and loss of appetite which developed 12 weeks previously. On admission, his body temperature was 38℃ and respiratory rate was 33/min. Pulmonary crackles were heard in both lower lung fields. The patient's PaO2 was 47.4 mmHg and O2 saturation was 88.9% on room air. Leukocyte count was 2,950/mm3 (58% neutrophils and 26.1% lymphocytes). Enzyme-linked immunosorbent assay for HIV was positive and this was confirmed by Western blot analysis. CD4 lymphocyte count was 14/mm3 and viral load was 13,000 copies/mL. The chest radiograph revealed ill defined ground glass opacities in both lungs with central and upper lobe predominance (Fig. 1). He was treated with trimethoprim-sulfamethoxazole (TMP/SMX), steroid, lopinavir/ritonavir, lamivudine, and zidovudine. Methenamine silver stain of bronchoalveolar lavage fluid was negative and the culture revealed no organisms. His general condition improved and fever abated on day 3 after the initiation of treatment. Therefore, we considered P. jirovecii to be the causative agent of pneumonia. The patient was then discharged after showing clinical improvement with three weeks of therapy.
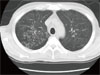
Five weeks later, he was admitted again with a 10-day history of cough and mucopurulent sputum. On admission, his vital signs were stable; body temperature was 37.4℃ and respiratory rate was 18/min. Laboratory results were as follows: PaO2, 82.1 mmHg and O2 saturation, 97% on room air; leukocyte count, 6,940/mm3 (78% neutrophils and 13.8% lymphocytes), erythrocyte sedimentation rate, 90 mm/h; and CD4 lymphocyte count, 101/mm3. Since crackles were heard on both lower lung fields, chest CT scan was taken and it showed multifocal clusters of ill defined small centrilobular nodular opacities with bronchial wall thickening and linear opacities in both lungs (Fig. 2). Treatment was initiated with TMP/SMX and IV cefuroxime (750 mg q 8 h) for presumed Pneumocystis jirovecii pneumonia with or without combined community acquired pneumonia. Bronchoscopy revealed diffuse acute inflammatory changes of the bronchial mucosa with abundant purulent bronchial secretions from both bronchi but methenamine silver stain for P. jirovecii was negative. However, the bronchial washing fluid culture yielded > 100,000 colonies/mL of C. macginleyi that was susceptible to vancomycin, gentamicin, and tobramycin, but resistant to penicillin, oxacillin, cephalothin, ciprofloxacin, and TMP/SMX. On day 6 after the initiation of antibiotic treatment, the patient developed fever and complained of shortness of breath. We therefore considered corynebacteria to be the causative agent of pneumonia and thus vancomycin was administered. He fully recovered after receiving a 14-day course of antibiotic therapy with vancomycin monotherapy.
Pulmonary infections are a leading cause of morbidity and mortality in persons with HIV infection [9, 10]. Following the introduction of highly active antiretroviral therapy and TMP/SMX chemoprophylaxis, the relative incidence of HIV-associated pneumonia has changed. Whereas the incidence of P. jirovecii pneumonia and tuberculosis declined, bacterial pneumonia have become the most frequently encountered HIV-associated opportunistic respiratory infections [11, 12]. Common respiratory tract bacterial pathogens (i.e., Streptococcus pneumoniae and Haemophilus influenzae) and less pathogenic bacteria, such as the coryneform bacterium, have been implicated as the causative agents [10-12].
Species of the genus Corynebacterium are widely distributed in the environment as normal inhabitants of soil and water. They are gram-positive, non-acid-fast, aerobic or facultatively anaerobic, asporogenous rods [1]. In the hospital setting, they may be cultured from the hospital environment, including surfaces of medical equipments [13], but they rarely account for clinical infections. During the past two decades, however, non-diphtheria Corynebacterium species have caused diseases in at risk populations, such as the immunocompromised patients with indwelling medical devices.
C. macginleyi is a member of lipophilic corynebacterial group of the genus Corynebacterium [3, 14]. The lipophilic corynebacteria are usually fastidious and grow more slowly than non-lipophilic strains, and they produce small colonies unless they are grown on media enriched with a significant amount of lipids which can be supplied by serum or Tween 80 [15]. The fact that they were exclusively cultured from eye materials suggested that the main habitat of this microorganism is in or around the eyes of the human body [2-5]. However, in 2002, Villanueva et al. presented the first case of infection in the urinary tract of a patient with a permanent bladder drainage catheter [16]. In 2003, two non-ocular infections with C. macginleyi were documented; one of them was an intravenous catheter-related infection and the other was infectious endocarditis [17, 18]. Recently, in 2008, septicemia caused by C. macginleyi was reported [19]. In various occasions patients suffering from C. macginleyi infections had undergone prior invasive procedures or were severely immunocompromised; many had underlying malignancies, AIDS, or were transplant recipients. Although the pathogenicity of this microorganism is not yet clear, it should be recognized as a potential cause of bacterial superinfections. Kwaszewska et al. showed that 75.6% of the lipophilic corynebacteria isolated as flora from human skin were able to form biofilms [20]. Therefore, biofilm formation seems to be a factor contributing to the virulence of corynebacteria, especially C. macginleyi. However, because little is known about the mechanism of biofilm formation by corynebacteria, further investigation is required.
Variety of antibiotic regimens have been used successfully in the treatment of extra-ocular cases: glycopeptides [16], beta-lactams [17], beta-lactams with aminoglycosides [18], and beta-lactams with clindamycin [19]. The susceptibility of the isolates in these cases appears to be different. Despite the limited number of isolates reported and the incomplete data available, the literature suggests that glycopeptide should be the preferred treatment for extra-ocular C. macginleyi infections [19].
The increasing number of reported infections with C. macginleyi in the immunocompromised patients suggests that infection with this pathogen is likely to become more widespread. Thus, the significance of positive cultures for C. macginleyi obtained from representative clinical samples in patients with signs and symptoms of bacterial infection should not be overlooked, and should be added to the list of organisms causing respiratory tract infections in this population.
Figures and Tables
References
1. von Graevenitz A, Pünter-Streit V, Riegel P, Funke G. Coryneform bacteria in throat cultures of healthy individuals. J Clin Microbiol. 1998. 36:2087–2088.

2. Tarr PE, Stock F, Cooke RH, Fedorko DP, Lucey DR. Multidrug-resistant Corynebacterium striatum pneumonia in a heart transplant recipient. Transpl Infect Dis. 2003. 5:53–58.

3. Krish G, Beaver W, Sarubbi F, Verghese A. Corynebacterium xerosis as a cause of vertebral osteomyelitis. J Clin Microbiol. 1989. 27:2869–2870.

4. Belmares J, Detterline S, Pak JB, Parada JP. Corynebacterium endocarditis species-specific risk factors and outcomes. BMC Infect Dis. 2007. 7:4.
5. Ferrer C, Ruiz-Moreno JM, Rodríguez A, Montero J, Alió JL. Postoperative Corynebacterium macginleyi endophthalmitis. J Cataract Refract Surg. 2004. 30:2441–2444.
6. Funke G, Pagano-Niederer M, Bernauer W. Corynebacterium macginleyi has to date been isolated exclusively from conjunctival swabs. J Clin Microbiol. 1998. 36:3670–3673.

7. Giammanco GM, Di Marco V, Priolo I, Intrivici A, Grimont F, Grimont PA. Corynebacterium macginleyi isolation from conjunctival swab in Italy. Diagn Microbiol Infect Dis. 2002. 44:205–207.
8. Joussen AM, Funke G, Joussen F, Herbertz G. Corynebacterium macginleyi: a conjunctiva specific pathogen. Br J Ophthalmol. 2000. 84:1420–1422.
9. Davis JL, Fei M, Huang L. Respiratory infection complicating HIV infection. Curr Opin Infect Dis. 2008. 21:184–190.

10. McKenzie R, Travis WD, Dolan SA, Pittaluga S, Feuerstein IM, Shelhamer J, Yarchoan R, Masur H. The causes of death in patients with human immunodeficiency virus infection: a clinical and pathologic study with emphasis on the role of pulmonary diseases. Medicine (Baltimore). 1991. 70:326–343.

11. Dufour V, Cadranel J, Wislez M, Lavole A, Bergot E, Parrot A, Rufat P, Mayaud C. Changes in the pattern of respiratory diseases necessitating hospitalization of HIV-infected patients since the advent of highly active antiretroviral therapy. Lung. 2004. 182:331–341.

12. Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990. 141:1356–1372.

13. Young VM, Meyers WF, Moody MR, Schimpff SC. The emergence of coryneform bacteria as a cause of nosocomial infections in compromised hosts. Am J Med. 1981. 70:646–650.

14. Riegel P, Ruimy R, de Briel D, Prévost G, Jehl F, Christen R, Monteil H. Genomic diversity and phylogenetic relationships among lipid-requiring diphtheroids from humans and characterization of Corynebacterium macginleyi sp. nov. Int J Syst Bacteriol. 1995. 45:128–133.

15. Funke G, von Graevenitz A, Clarridge JE 3rd, Bernard KA. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997. 10:125–159.

16. Villanueva JL, Domínguez A, Ríos MJ, Iglesias C. Corynebacterium macginleyi isolated from urine in a patient with a permanent bladder catheter. Scand J Infect Dis. 2002. 34:699–700.

17. Dobler G, Braveny I. Highly resistant Corynebacterium macginleyi as cause of intravenous catheter-related infection. Eur J Clin Microbiol Infect Dis. 2003. 22:72–73.

18. Pubill Sucarrat M, Martinez-Costa X, Sauca Subias G, Capdevila Morell JA. Corynebacterium macginleyi as an exceptional cause of endocarditis: a case report. An Med Interna. 2003. 20:654–655.
19. Villamil-Cajoto I, Rodríguez-Otero L, García-Zabarte MA, Aguilera-Guirao A, García-Riestra C, Regueiro BJ, Villacián-Vicedo MJ. Septicemia caused by Corynebacterium macginleyi: a rare form of extraocular infection. Int J Infect Dis. 2008. 12:333–335.

20. Kwaszewska AK, Brewczyńska A, Szewczyk EM. Hydrophobicity and biofilm formation of lipophilic skin corynebacteria. Pol J Microbiol. 2006. 55:189–193.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download