Abstract
Toxoplasmosis is a rare but fatal complication in hematopoietic stem cell transplant recipients, usually associated with allogeneic hematopoietic stem cell transplantation (HSCT). We report a case of cerebral toxoplasmosis in a patient with multiple myeloma, following tandem autologous stem cell transplantation. A 55-year-old Korean male presented with weakness in both legs that had progressed to both arms. A magnetic resonance imaging scan of the brain revealed multiple, variable-sized ring-enhancing lesions with surrounding edema in the cerebral hemispheres and brain stem. Stereotactic biopsy revealed bradyzoites of Toxoplasma gondii in the brain tissue. The patient received trimethoprim-sulfamethoxazole, followed by pyrimethamine and sulfadiazine, accompanying treatment for progressive multiple myeloma. Cerebral toxoplasmosis should be considered as one of the differential diagnoses in patients with neurologic signs following autologous HSCT.
Toxoplasmosis is an opportunistic infection caused by Toxoplasma gondii, a ubiquitous obligate intracellular protozoan. T. gondii causes encephalitis, pneumonitis, and disseminated infection in immunocompromised hosts, and leads to life-threatening infections in some patients (1). Toxoplasmosis is a rare complication in hematopoietic stem cell transplantation (HSCT) recipients. The incidence of toxoplasmosis after HSCT is 0.3-7.6%, with higher rates in countries where toxoplasmosis is more prevalent (2). Toxoplasmosis is usually associated with allogeneic HSCT but can also be associated with autologous HSCT (3). Toxoplasmosis following HSCT is usually the result of reactivation of latent infection rather than primary infection (2, 4-6). Diagnosing toxoplasmosis following HSCT is often difficult, because the symptoms and signs are nonspecific. Therefore, it is important to take toxoplasmosis into account as one of the differential diagnoses in febrile HSCT recipients (1).
The incidence of toxoplasmosis following HSCT in South Korea is not known, and this is the second report on cerebral toxoplasmosis in HSCT recipients and the first report in autologous HSCT recipients. We report a case of cerebral toxoplasmosis which presented as Guillain-Barre syndrome (GBS) and was diagnosed by brain biopsy.
A 55-year-old Korean male was admitted to Seoul National University Hospital because of fever. Since pneumonia was clinically suspected, he received cefotaxime 6 g/day for 7 days and azithromycin 500 mg/day for 4 days and the fever subsided. He was then discharged with moxifloxacin which was to be taken 400 mg/day for 5 days. Ten days after discharge, he felt weakness of the left leg, and was not able to stand up without taking another person's arm. Then, five days later, he felt weakness on the right leg; three days later, the weakness had progressed to both arms and he was unable to hold a spoon. He was readmitted to our hospital.
Approximately 10 months before admission, the patient had lost body weight and attended another hospital. The results of complete blood count and urinalysis revealed anemia and proteinuria. He was diagnosed with multiple myeloma after a bone marrow examination. He was referred to our hospital and was given thalidomide 100 mg/day and dexamethasone 40 mg/day for 4 days, every 4 weeks for five months. However, multiple myeloma did not respond to this treatment. Approximately 5 months before admission, he received cyclosphosphamide 5,350 mg for hematopoietic stem cell collection. One month later, following conditioning with melphalan, he underwent autologous HSCT. On the 85th day of HSCT, the monoclonal protein had decreased but a bone marrow biopsy revealed a partial response. Three months after the first HSCT, he underwent a second, tandem, autologous HSCT. To prevent infection during neutropenic state, he received fluconazole and ciprofloxacin, but he did not received trimethoprim-sulfamethoxazole (TMP-SMX).
On admission, his sensation in both his legs and distal arms had decreased. He did not have fever, pain, shortness of breath, dysarthria, dysphagia, and urinary or bowel dysfunction. On examination, the patient was not in acute distress. On neurologic examination, the motor strength of the upper extremities was reduced to IV+/V in the upper arms, II+ in the finger adductors and abductors, IV+ in the finger flexors, and I+ in the finger extensors. Motor strength of the lower extremities was reduced to II+ in the hip flexors and extensors, I+ in the knee flexors, III+ in the knee extensors, and I+ in the ankle dorsiflexors, extensors and toes. Deep tendon reflexes were hypoactive in all four extremities. Both flexor responses were present. Examinations of sensation revealed hypesthesia in all four extremities. He was alert and oriented, with normal attention and speech, and the cranial nerves were normal.
The results of a complete blood count were as follows: white blood cells, 3,000/µL (segmented neutrophils 68.1% and lymphocytes 18.6%); hemoglobin, 10.2 g/dL; platelets, 181×103/µL. The results for serum glucose, electrolytes, urea nitrogen, and creatinine were normal. The result of a cerebrospinal fluid (CSF) examination was as follows: opening pressure, 9.5 cmH2O; lymphocytes, 5/mm3; protein, 157.5 mg/dL; and glucose, 81 mg/dL. Gram staining and culture of CSF revealed no microorganisms. Polymerase chain reactions (PCR) for cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus (EBV), and Mycobacterium tuberculosis were not preformed. Antigen test for cryptococcus was not performed as well. An MRI scan of the whole spinal column indicated diffuse involvement of multiple myeloma in the bone marrow and disc extrusion of L1-2 and L2-3, without evidence of epidural mass formation. An MRI scan of the brain was not performed. Sensory and motor nerve conduction studies of the bilateral upper and lower extremities revealed little or no conduction velocity and were consistent with sensorimotor polyneuropathy.
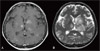
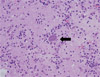
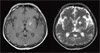
Since GBS was suspected, he received intravenous immunoglobulin 21 g/day for 10 days and prednisolone 1 mg/kg/day for a month. Motor strength recovered to III+ in both legs. However, two months later, diplopia, dysarthria, drowsy mentality, and urinary incontinence developed. The results of cerebrospinal fluid (CSF) examination were as follows: opening pressure, 11 cmH2O; lymphocytes, 9/mm3; protein, 125.8 mg/dL; and glucose, 78 mg/dL. Gram staining and culture of CSF were negative for microorganisms. PCR for CMV, EBV, HSV, enterovirus, and M. tuberculosis were all negative, and cryptococcal antigen assay was negative as well. PCR for T. gondii could not be performed because it was not available at our center. An MRI scan of the brain revealed multiple and variable-sized ring-enhancing lesions with surrounding edema in the bilateral cerebral hemispheres and brain stem (Fig. 1). Stereotactic biopsy revealed bradyzoites of T. gondii in the brain tissue (Fig. 2), and anti-T. gondii IgG antibody (Abbott, Wiesbaden, Germany) was positive with a titer exceeding 300 IU/mL. Anti-T. gondii IgG antibody was not performed before HSCT. He received TMP-SMX 480-2,400 mg/day for 6 days followed by pyrimethamine 100 mg/day and sulfadiazine 4 g/day for 6 weeks. After the treatment, motor strength in the left arm recovered to IV+ and mental status improved. An MRI scan of the brain performed after treatment for 55 days and showed improvement of the multifocal enhancing granulomatous lesions, with decreased size and number of enhancing lesions and a reduction in the amount of surrounding edema compared to the previous examination (Fig. 3). At that time, the results of a complete blood count were as follows: white blood cells, 2,670/µL (segmented neutrophils 71% and lymphocytes 11%); hemoglobin, 9.3 g/dL; platelets, 229×103/µL.
Three months after the tandem HSCT, serum monoclonal protein was not detected. However, four months after the tandem HSCT, serum monoclonal protein (M-protein) was detected as 0.389 g/dL and five month later, M protein increased to 0.843 g/dL. After eight-week of treatment for toxoplasmosis, he received cyclosphosphamide 400 mg/day and dexamethasone 40 mg/day for 4 days. In spite of four cycles of cyclosphosphamide/dexamethasone therapy for 5 months, his disease progressed. Then, he received bortezomib 2.1 mg/day on day 1, 4, 8, and 11 and dexamethasone 20 mg/day on day 1, 2, 4, 5, 8, 9, 11, and 12, on an each cycle, every 3 weeks. He received 3 cycles of bortezomib and dexamethasone at our hospital for 2 months and then he continued chemotherapy at another hospital.
Maintenance therapy for cerebral toxoplasmosis with pyrimethamine 25 mg/day and sulfadiazine 2 g/day were continued for one year while he was treated for progressive multiple myeloma with cyclosphosphamide/dexamethasone and bortezomib/dexamethasone. After a year of maintenance therapy, he became able to walk holding another person's hand. During the follow up at our hospital, neurologic signs and symptoms did not worsen. After 1 year of maintenance therapy for cerebral toxoplasmosis, maintenance therapy stopped and he received supportive care for rehabilitation at other hospital. MRI scans were taken every 3 months during the maintenance therapy and showed that enhancing nodules were reduced compared with the scan at the time of initial diagnosis.
Toxoplasmosis is a rare opportunistic infection in HSCT recipients and can be fatal. The incidence of toxoplasmosis in HSCT recipients varies geographically, because seropositivity differs between geographic areas (1). In France, seropositivity among the population is 50-80% and the incidence of toxoplasmosis in HSCT recipients is 5%. In the USA, overall seropositivity was 22.8% in a national survey between 1988 and 1994, and the incidence of toxoplasmosis in HSCT recipients is 0.3% (1, 7). In Korea, seropositivity of the general population is reported to be 0.8-12.9% (8-10). In a recent study in Daejeon, seropositive rate was 6.6-6.7%, which is not as high as that in western countries (11).
In Korea, there have been several cases of cerebral toxoplasmosis in patients with acquired immune deficiency syndrome (12, 13); however, there was one case of cerebral toxoplasmosis with retinochoroiditis in HSCT recipients. In our HSCT center, 918 patients received allogeneic or autologous HSCT between 1985 and 2006. This is the first confirmed case of cerebral toxoplasmosis out of 49 autologous HSCT recipients (2%) in our center.
Toxoplasmosis following HSCT usually occurs after reactivation of latent infection. Toxoplasmosis occurs mainly in allogeneic transplant recipients, but few cases of cerebral toxoplasmosis have been reported in autologous transplant recipients. In allogeneic transplant recipients, toxoplasmosis occurs in patients with severely impaired cell-mediated immunity that experience severe graft versus host disease (GVHD) or require intensive immunosuppressive therapy (14, 15). In autologous transplant recipients, the risk of toxoplasmosis is associated with underlying malignancy. Like our case, multiple myeloma itself is related to impaired humoral immunity and patients need to receive additional therapy (16). He was exposed to multiple chemotherapeutic agents and his immune system may have been affected more profoundly than in routine autologous HSCT. In addition to this, he received a high dose of corticosteroid for one month because GBS was suspected. Immunosuppressive therapy including corticosteroid interferes with macrophage or T cell function and predisposes the recipients to active infection. Cellular immunity such as CD8+ lymphocyte with interferon-gamma plays a critical role in the defense against the toxoplasmosis (17).
In autologous setting, pre-transplant serology for T. gondii is not performed routinely. In a review of 52 HSCT centers in different areas, pre-transplant serology for T. gondii was found to be performed in 82% (37/45) of the centers in allogeneic HSCT, but in only 52% (27/52) in autologous setting (5). In our center, pre-transplant serology for T. gondii is recommended in both recipients and donors in case of allogeneic HSCT. Routine pre-transplantation serologic testing should be considered in tandem autologous HSCT recipients, because of the following reasons. First, even in the 1st autologous HSCT, recipients receive chemotherapeutic agents multiple times. Second, after HSCT, serologic test does not play a role in the diagnosis of toxoplasmosis, because IgG production is low in HSCT recipients (17). Third, TMP-SMX prophylaxis, which is also effective for Pneumocystis pneumonia, is effective for toxoplasmosis; however, in autologous HSCT, recipients usually do not receive TMP-SMX prophylaxis (18).
Diagnosis of toxoplasmosis following HSCT is often difficult, because the symptoms and signs are nonspecific (17). Moreover, the recipient's immune response is unpredictable due to immunosuppressive therapy (1). High fever is often the earliest sign and there are often nonspecific signs in the involved organs. Therefore, in febrile HSCT recipients with neurologic signs, prompt diagnostic workup is important (19). In our case, the patient presented with fever 21 days before developing motor weakness and the fever subsided after empirical antibiotic therapy. When motor weakness was present, nerve conduction studies were consistent with sensorimotor polyneuropathy. GBS was suspected, although sensorimotor polyneuropathy is not specific for GBS. Thalidomide is frequently reported to cause a length-dependent axonal neuropathy. Moreover, increased toxicity in the combination therapy of thalidomide and dexamethasone was reported recently (20). Considering the impaired immunity due to tandem autologous HSCT, brain MRI should have been performed to evaluate the cerebral lesions in our case. He received corticosteroids and the fever was masked and the edema around the lesions decreased in the early phase of the clinical course.
In conclusion, toxoplasmosis is a relatively rare opportunistic infection in HSCT recipients. Few cases have been reported in Korea, but it can be a fatal complication in HSCT. A high suspicion is important to diagnose cerebral toxoplasmosis when HSCT recipient presents with fever and neurologic signs, such as weakness of motor strength.
Figures and Tables
Figure 1
MRI scans of the brain at the time of diagnosis of cerebral toxoplasmosis. Multiple variable-sized ring-enhancing lesions with surrounding edema are shown in the bilateral cerebral hemispheres and brainstem. Lesions of T1 high intensity (A) and T2 low intensity (B) suggest focal hemorrhage.
Acknowledgements
This case was presented, in part, at the 17th International Congress for Tropical Medicine and Malaria (Jeju, Korea, 2008).
We are grateful to Dr. Jongyoun Yi, for critical review of the manuscript.
References
1. Mele A, Paterson PJ, Prentice HG, Leoni P, Kibbler CC. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant. 2002. 29:691–698.

2. Kotton CN. Zoonoses in solid-organ and hematopoietic stem cell transplant recipients. Clin Infect Dis. 2007. 44:857–866.

3. Pagano L, Trapè G, Putzulu R, Caramatti C, Picardi M, Nosari A, Cinieri S, Caira M, Del Favero A. GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto)-Infection Program. Toxoplasma gondii infection in patients with hematological malignancies. Ann Hematol. 2004. 83:592–595.

4. Roemer E, Blau IW, Basara N, Kiehl MG, Bischoff M, Günzelmann S, Kirsten D, Sanchez H, Wocker EL, Fauser AA. Toxoplasmosis, a severe complication in allogeneic hematopoietic stem cell transplantation: successful treatment strategies during a 5-year single-center experience. Clin Infect Dis. 2001. 32:E1–E8.

5. Martino R, Bretagne S, Rovira M, Ullmann AJ, Maertens J, Held T, Deconinck E, Cordonnier C. Toxoplasmosis after hematopoietic stem transplantation. Report of a 5-year survey from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2000. 25:1111–1114.

6. Edvinsson B, Lundquist J, Ljungman P, Ringdén O, Evengård B. A prospective study of diagnosis of Toxoplasma gondii infection after bone marrow transplantation. APMIS. 2008. 116:345–351.

7. Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol. 2001. 154:357–365.

8. Lee YH, Noh HJ, Hwang OS, Lee SK, Shin DW. Seroepidemiological study of Toxoplasma gondii infection in the rural area Okcheon-gun, Korea. Korean J Parasitol. 2000. 38:251–256.

9. Yang HJ, Jin KN, Park YK, Hong SC, Bae JM, Lee SH, Choi HS, Hwang HS, Chung YB, Lee NS, Nam HW. Seroprevalence of toxoplasmosis in the residents of Cheju island, Korea. Korean J Parasitol. 2000. 38:91–93.

10. Song KJ, Shin JC, Shin HJ, Nam HW. Seroprevalence of toxoplasmosis in Korean pregnant women. Korean J Parasitol. 2005. 43:69–71.

11. Shin DW, Cha DY, Hua QJ, Cha GH, Lee YH. Seroprevalence of Toxoplasma gondii Infection and characteristics of sero-positive patients in general hospitals in Daejeon, Korea. Korean J Parasitol. 2009. 47:125–130.

12. Kim BH, Lee SI, Lee CH, Cha SH, Lee TH, Lee SH, Chung JS, Cho GJ. A case of cerebral toxoplasmosis in a patient with acquired immune defeciency syndrome. Infect Chemother. 2004. 36:181–184.
13. Nam TS, Seo KS, Lee KI, Kim YS, Hong JH, Kim GH, Jeong JH, Chu HJ, Park SK, Seoung NH, Jung JS, Cho GJ. The clinical study of hematoimmunologic features and opportunistic infections of patients with AIDS. Korean J Med. 1997. 52:15–23.
14. Chandrasekar PH, Momin F. Bone Marrow Transplant Team. Disseminated toxoplasmosis in marrow recipients: a report of three cases and a review of the literature. Bone Marrow Transplant. 1997. 19:685–689.

15. de Medeiros BC, de Medeiros CR, Werner B, Loddo G, Pasquini R, Bleggi-Torres LF. Disseminated toxoplasmosis after bone marrow transplantation: report of 9 cases. Transpl Infect Dis. 2001. 3:24–28.

16. Anaissie E, Nucci M. Bowden RA, Ljungman P, Paya CV, editors. Risk and epidemiology of infections after autologous hemopoietic stem cell transplantation. Transplant infections. 2003. 2nd ed. Philadelphia: Lippincott Winlliams & Wilkins;39–50.
17. Martino R. Bowden RA, Ljungman P, Paya CV, editors. Toxoplasmosis after hematopoietic stem cell transplantation. Transplant Infections. 2003. 2nd ed. Philadelphia: Lippincott Winlliams & Wilkins;535–540.

18. López-Duarte M, Insunza A, Conde E, Iriondo A, Mazorra F, Zubizarreta A. Cerebral toxoplasmosis after autologous peripheral blood stem cell transplantation. Eur J Clin Microbiol Infect Dis. 2003. 22:548–550.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download