Abstract
Papillary carcinomas account for 85~90% of all thyroid cancers, with the tumor size considered an important prognostic factor. As the use of high-resolution ultrasonography and fine needle aspiration biopsy have increased, the diagnosis of papillary microcarcinomas of the thyroid gland; defined by the World Health Organization as being less than 1 cm in diameter, has increased. They are generally associated with an excellent prognosis, with distant metastasis being extremely rare. They usually remain clinically silent until their incidental histological diagnosis by autopsy or surgical material. The incidence discovered at autopsy varies between 3 and 36%. Cervical lymph node metastases from papillary microcarcinomas have often been discovered, which may be the first and sole manifestation of the disease, without clinical suspicion of a thyroid tumor. Herein, the case of a papillary thyroid microcarcinoma, diagnosed after a total thyroidectomy due to its first presentation as a contralateral cervical lymph node metastasis, without evidence of a clinical thyroid tumor, is described.
Figures and Tables
Fig. 1
Neck ultrasonogram. A 30×19×14 mm, well demarcated, lobulating mass with cystic and solid component was detected in the left upper posterior cervical triangle.
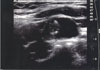
Fig. 2
Computed tomography of the neck after removal of cervical lymph node (left). No discrete mass in both lobes of the thyroid gland.
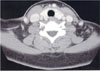
Fig. 3
Histology of the excised neck mass shows compacted of the tumor cells, characteristics of papillary thyroid carcinoma, and is surrounded by lymphocytic infiltration suggestive of cervical lymph node metastasis. (H-E stain, A: ×100, B: ×400)
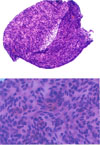
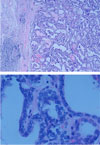
Fig. 4
A. After total thyroidectomy, the characteristic papillary structures and fibrovascular cores were found in tumor tissue. (H-E stain, ×50)
B. The lining epithelium shows atypical nucleus characteristics of papillary carcinoma including nuclear grooving and intranuclear inclusion body. (H-E stain, ×400)
References
1. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004. 135:139–148.
2. Hedinger C. Hedinger C, Williams ED, Sobin LH, editors. Histologic typing of thyroid tumors. International Histological Classification of Tumors. 1988. 2nd ed. Berlin: Springer-Verlag;1–67.
3. Chow SM, Law SC, Au SK, Mang O, Yau S, Yuen KT, Lau WH. Changes in clinical presentation, management and outcome in 1348 patients with differentiated thyroid carcinoma: experience in a single institute in Hong Kong, 1960~2000. Clin Oncol. 2003. 15:329–336.
4. Noguchi S. Differentiated thyroid carcinomas in Japan: our experience and review of the literature. Thyroidol Clin Exp. 1998. 10:41–50.
5. Chow SM, Law SCK, Chan JKC, Aun SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid: prognostic significance of lymph node metastases and multifocality. Cancer. 2003. 98:31–40.
6. Monchik JM, Petris G, Crea C. Occult papillary carcinoma of the thyroid presenting as a cervical cyst. Surgery. 2001. 129:429–432.
7. Pellegriti G, Scollo C, Lumera G, Regalbuto C, Vigneri R, Belfiore A. Clinical behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: study of 299 cases. J Clin Endocrinol Metab. 2004. 89:3713–3720.
8. Carpi A, Nicolini A, Casara D, Rubello D, Pelizzo MR. Nonpalpable thyroid carcinoma. Am J Clin Oncol. 2003. 26:232–235.
9. Al-Dhabri S, Al-Sebeih K, Hier MP, Black MJ. An aggressive approach to the surgical management of suspicious thyroid nodules. J Otolaryngo. 2001. 30:203–207.
10. Flanagan D, Gibb P, Skene A, McCutcheon J, Armitage M. What should we do? Papillary thyroid carcinoma in a lymph node but normal thyroid tissue-how should we proceed? Eur J Surg Oncol. 2000. 26:177–180.
12. Davis P, Perrier N, Adler L, Levine E. Incidental thyroid carcinoma identified by positron emission tomography scanning obtained for metastatic evaluation. Am Surg. 2001. 67:582–584.
13. Cohen M, Arslan N, Dehdashti F, Doherty G, Lairmore T, Brunt M, Moley J. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001. 130:941–946.
14. Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992. 112:1139–1147.
15. Noguchi S, Yamashita H, Murakami N, Nakayama I, Masakatsu T, Kawamoto H. Small carcinomas of the thyroid. a long-term follow-up of 867 patients. Arch Surg. 1996. 131:187–191.
16. Yamashita H, Noguchi S, Murakami N, Toda M, Uchino S, Watanabe S, Kawamoto H. Extracapsular invasion of lymph node metastasis. a good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999. 86:842–849.
17. Furlan JC, Bedard Y, Rosen IB. Biologic basis of the management of microsocopic, occult well-differentiated thyroid cancer. Surgery. 2001. 130:1050–1054.
18. Coleman S, Smith J, Burkey B, Day T, Page R, Netterville J. Long standing lateral neck mass as the initial manifestation of well-differentiated thyroid carcinoma. Laryngoscope. 2000. 110:204–209.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download