1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853. PMID:
9742976.
2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589. PMID:
18784090.

3. Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC Jr, Peterson K, et al. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012; 35:409–414. PMID:
22179956.

4. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012; 35:1897–1901. PMID:
22699297.

5. Lee JH, Choi Y, Jun C, Hong YS, Cho HB, Kim JE, et al. Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul). 2014; 29:112–121. PMID:
25031883.

6. Williams SA, Shi L, Brenneman SK, Johnson JC, Wegner JC, Fonseca V. The burden of hypoglycemia on healthcare utilization, costs, and quality of life among type 2 diabetes mellitus patients. J Diabetes Complications. 2012; 26:399–406. PMID:
22699113.

7. Kim JT, Oh TJ, Lee YA, Bae JH, Kim HJ, Jung HS, et al. Increasing trend in the number of severe hypoglycemia patients in Korea. Diabetes Metab J. 2011; 35:166–172. PMID:
21738899.

8. Yun JS, Ko SH, Ko SH, Song KH, Ahn YB, Yoon KH, et al. Presence of macroalbuminuria predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care. 2013; 36:1283–1289. PMID:
23248198.
9. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013; 36:1384–1395. PMID:
23589542.

10. Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009; 58:360–366. PMID:
19056608.
11. Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. Diabetes Res Clin Pract. 1994; 24(Suppl):S261–S268. PMID:
7859616.

12. Yamanouchi T, Minoda S, Yabuuchi M, Akanuma Y, Akanuma H, Miyashita H, et al. Plasma 1,5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes. 1989; 38:723–729. PMID:
2656341.

13. Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003; 5:355–363. PMID:
12828817.

14. Kim MJ, Jung HS, Hwang-Bo Y, Cho SW, Jang HC, Kim SY, et al. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2013; 50:505–510. PMID:
21688018.

15. Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, et al. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006; 29:1214–1219. PMID:
16731998.

16. Murata GH, Hoffman RM, Shah JH, Wendel CS, Duckworth WC. A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: The Diabetes Outcomes in Veterans Study (DOVES). Arch Intern Med. 2004; 164:1445–1450. PMID:
15249354.
17. Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005; 28:2948–2961. PMID:
16306561.
18. Yun JS, Ko SH. Severe hypoglycemia in patients with diabetes. Diabetes Metab J. 2012; 36:273–274. PMID:
22950057.

19. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995; 18:517–522. PMID:
7497862.

20. American Diabetes Association. (6) Glycemic targets. Diabetes Care. 2015; 38(Suppl):S33–S40. PMID:
25537705.
21. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011; 13:921–928. PMID:
21714681.

22. Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab (Seoul). 2015; 30:167–174. PMID:
26194076.

23. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006; 29:2433–2438. PMID:
17065680.

24. Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med. 2007; 24:753–758. PMID:
17459094.

25. Saisho Y, Tanaka C, Tanaka K, Roberts R, Abe T, Tanaka M, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes. 2015; 9:290–296. PMID:
25456706.

26. Kishimoto M, Yamasaki Y, Kubota M, Arai K, Morishima T, Kawamori R, et al. 1,5-Anhydro-D-glucitol evaluates daily glycemic excursions in well-controlled NIDDM. Diabetes Care. 1995; 18:1156–1159. PMID:
7587851.

27. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998; 21:1870–1875. PMID:
9802735.

28. Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013; 347:f4533. PMID:
23900314.

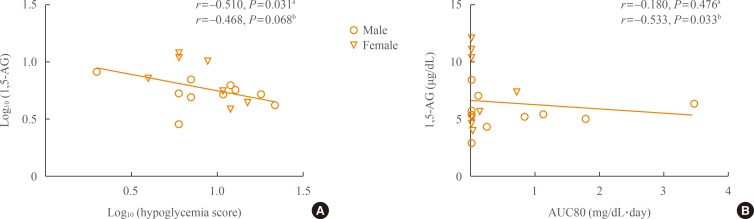
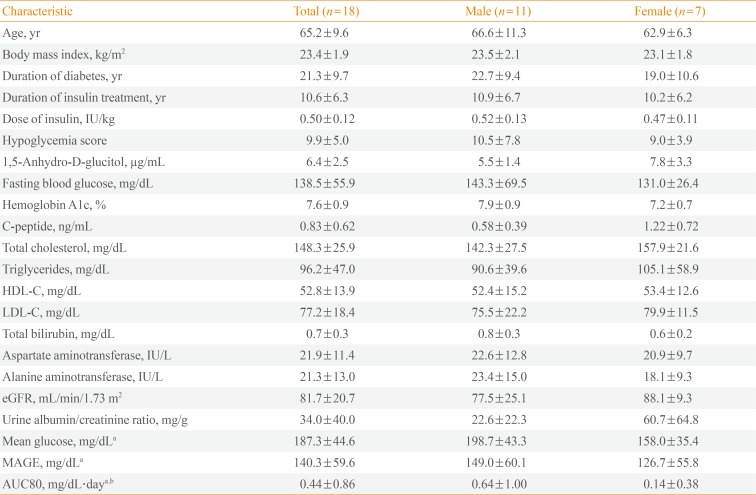
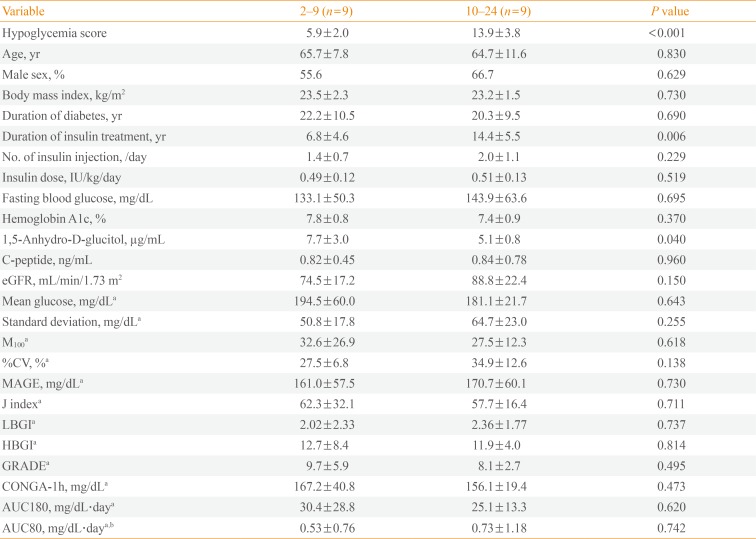
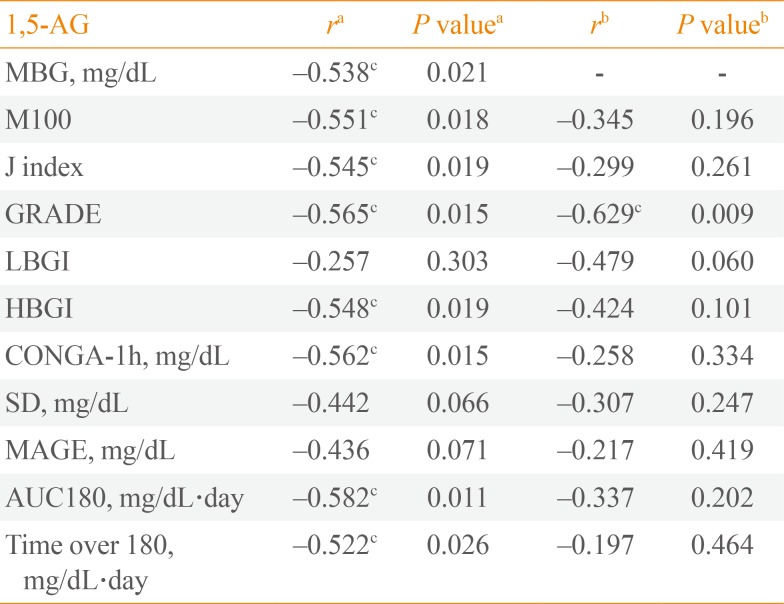




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download