Abstract
Kisspeptin signaling at the gonadotropin-releasing hormone (GnRH) neuron is now relatively well characterized and established as being critical for the neural control of fertility. However, kisspeptin fibers and the kisspeptin receptor (KISS1R) are detected throughout the brain suggesting that kisspeptin is involved in regulating the activity of multiple neuronal circuits. We provide here a review of kisspeptin actions on neuronal populations throughout the brain including the magnocellular oxytocin and vasopressin neurons, and cells within the arcuate nucleus, hippocampus, and amygdala. The actions of kisspeptin in these brain regions are compared to its effects upon GnRH neurons. Two major themes arise from this analysis. First, it is apparent that kisspeptin signaling through KISS1R at the GnRH neuron is a unique, extremely potent form or neurotransmission whereas kisspeptin actions through KISS1R in other brain regions exhibit neuromodulatory actions typical of other neuropeptides. Second, it is becoming increasingly likely that kisspeptin acts as a neuromodulator not only through KISS1R but also through other RFamide receptors such as the neuropeptide FF receptors (NPFFRs). We suggest likely locations of kisspeptin signaling through NPFFRs but note that only limited tools are presently available for examining kisspeptin cross-signaling within the RFamide family of neuropeptides.
It is now just over a decade since the key importance of kisspeptin signaling was identified for human fertility [123]. A very substantial international research effort over this time has identified that kisspeptin activates kisspeptin receptors (KISS1R) on gonadotropin-releasing hormone (GnRH) neurons to control puberty onset and subsequent fertility in all mammals [4567]. However, it has always been apparent that kisspeptin signaling occurs in many other body organs [89] in addition to regions of the brain not thought to be involved in controlling the activity of GnRH neurons [10111213141516]. Furthermore, recent studies have begun to highlight cross-talk amongst the various RFamide receptors and ligands, including the ability of kisspeptin to activate neuropeptide FF (NPFF) receptors (NPFFR) [17181920]. Thus, kisspeptin appears to be involved in regulating multiple neuronal networks within the forebrain. We provide here a review of the effects of kisspeptin on the activity of neurons throughout the forebrain. We start with a very brief review of the discovery of kisspeptin and its effects on the GnRH neurons, before concentrating upon the actions and potential roles of kisspeptin signaling in non-GnRH neuronal networks.
While searching for melanoma metastasis-suppressor genes, Lee and coworkers [21] identified a novel cDNA that was only expressed in nonmetastatic human melanoma cell lines and, being located in Hershey (PA, USA), named it "KiSS-1" after the Hershey's Kissess chocolate. Four N-terminal truncated and C-terminal amidated peptides with 54, 14, 13, and 10 amino acids, derived from a 145 amino acid protein, were subsequently isolated from human placenta and designated as kisspeptin-54 (also named as metastin [22]), kisspeptin-14, kisspeptin-13, and kisspeptin-10 [1213222324].
The orphan G-protein-coupled receptor 54 (GPR54) was identified as being the cognate receptor for kisspeptin by three independent laboratories in 2001 [121322]. As the various kisspeptin peptides bind with equal affinity and efficacy to GPR54 [121322], most research has been undertaken using kisspeptin-10. Unless otherwise stated, "kisspeptin" will be used here to represent kisspeptin-10. More recently, it has been suggested that GPR54 should be re-named KISS1R [2526] and this review will use this nomenclature. It is also important to note that multiple KISS and KISSR homologues are found throughout evolution but only KISS1 and KISS1R remain in mammals [2728].
Kisspeptin was discovered to be the most potent activator of GnRH neuron excitability in 2005 [29]. Since then several research groups have examined in great detail the cellular mechanisms through which kisspeptin activates GnRH neurons and the electrophysiological properties of kisspeptin neurons [30313233]. In essence, endogenous [34] and exogenous [2935363738] kisspeptin acts directly upon KISS1R expressed on the cell body/proximal dendrites of most GnRH neurons with low nanomolar efficacy (EC50=3 to 5 nM) [3738] to depolarize the neuron in vitro and in vivo [39] in mice (Fig. 1A). The peculiar features of this response are that, for a neuropeptide, kisspeptin is extraordinarily potent and its effects are long-lasting but rarely repeatable in the brain slice preparation [31].
Experiments in brain slices demonstrate that kisspeptin exerts its potent stimulatory actions upon GnRH neurons largely by modulating the activity of potassium and non-selective cationic channels [35363738]. Kisspeptin closes inward rectifier potassium channels to depolarize GnRH neurons [3536373840] and available evidence indicates that this occurs through Kiss1r-Gαq-phospholipase C (PLC) signalling that may involve phosphatidylinositol-4,5-bisphosphate [363841]. Kisspeptin also allows the influx of cations into GnRH neurons by opening a transient receptor potential cationic 4 channel (TRPC4) [363841]. This also results from Kiss1r-Gαq activation of PLC that, in this case, dissociates PIP2 from the TRPC4 channel to potentiate its activity while also activating tyrosine kinase cSrc to phosphorylate and activate TRPC4. Thus Kiss1r-Gαq-PLC appears to be the primary signalling pathway orchestrating the activity of multiple ion channels to evoke potent excitation in GnRH neurons.
Interestingly, kisspeptin acts at multiple different locations along the GnRH neuron to modulate its electrical activity and the secretion of GnRH. For example, kisspeptin puffed onto GnRH neuron dendrites located outside the blood-brain barrier in the organum vasculosum of the lamina terminalis can also activate GnRH neuron firing [42]. Similarly, kisspeptin applied to the distal dendritic projections of GnRH neurons within and around the median eminence is also able to evoke the secretion of GnRH [43444546]. The signalling pathways mediating kisspeptin's actions on GnRH neuron dendritic projections is unclear at present but likely involves release of calcium from both internal and external sources [44].
Kisspeptin may also exert indirect actions on GnRH neurons with both gamma-aminobutyric acid (GABA)-glutamate and nitric oxide signalling being modified in the vicinity of the GnRH neuron cell bodies under some circumstances [3747]. However, the functional significance of these indirect actions is unclear as the direct effects of kisspeptin on GnRH neurons are the critical site of action for both the electrophysiological [5] and reproductive actions of kisspeptin [54849].
Finally, it is worth noting that the so-called NPFF receptor antagonist adamantylcarbonyl-arginyl-phenylalaninamide (RF9) [50] has recently been demonstrated to directly activate GnRH neuron firing in a KISS1R-dependent manner [51] and also shown to bind to KISS1R in in vitro assays [5253]. This indicates that previous studies reporting dramatic effects of RF9 on the secretion of GnRH [54] and gonadotropins [555657] did not result from inhibition of NPFF receptors.
The distribution of kisspeptin-immunoreactive fibers has been mapped in many mammals [58] although most information is available for mice and rats [1059606162]. In general, kisspeptin fibers are located in greatest numbers throughout the preoptic area and hypothalamus. This includes most of the preoptic area sub-nuclei, paraventricular, dorsomedial and arcuate (ARN) nuclei, and the lateral and posterior hypothalamic areas. Notably, few kisspeptin fibers are found within the ventromedial and supraoptic nuclei (SON) and species differences exist with respect to the suprachiasmatic nucleus. Outside of the hypothalamus, kisspeptin fibers are located in the septum, subfornical organ, bed nucleus of the stria terminalis, medial amygdala, anterior and paraventicular nucleus of thalamus and preaquaductal gray and locus coeruleus of the brainstem [1059606162636465]. Anterograde and retrograde tracing studies have shown that both of the principal kisspeptin neuron populations located in the preoptic area and ARN contribute to these projections, with those from the ARN being more widely dispersed throughout the brain [606364].
The absence of specific antisera for KISS1R has resulted in much less information being available on the distribution of the KISS1R in the brain. Early studies examining broad brain regions indicated that KISS1R mRNA was expressed widely throughout the human brain [1213]. An early in situ study in rats also indicated that Kiss1r mRNA was expressed in many different brain regions including the pons, midbrain, thalamus, hypothalamus, hippocampus, amygdala, cortex, frontal cortex, and striatum [14]. The most detailed maps of KISS1R-expressing cells have been undertaken in genetically modified mice that have LacZ knocked into the Kiss1r locus [15] and in a recent rat Kiss1r mRNA study [16]. These studies showed high levels of KISS1R in the GnRH neuron population alongside expression in some of the brain regions known to have kisspeptin fibers in the mouse. In addition, many brain regions that do not have detectable kisspeptin-immunoreactive fibers were observed to express KISS1R; for example the hippocampal dentate gyrus, supramammillary nuclei and various thalamic nuclei [15]. It is important to note that, at present, the reported distribution patterns of KISS1R appear highly discordant between species. Some of this may be methodological but differences in certain regions may reflect true species variation. For example, most evidence indicates that there is very little or no KISS1R in the arcuate nucleus of the mouse [1543] although it is present in the rat [16] and primate [66].
Together, these neuroanatomical studies indicate that kisspeptin signaling occurs at multiple locations within the brain and that there is only moderate overlap between the locations of the receptor and ligand. This suggests the presence of other ligands for KISS1R and, equally, that kisspeptin may act at receptors other than KISS1R.
The mammalian RFamide peptides share a common carboxy terminal Arg-Phe-amide motif and are classified into five distinct families; RFamide-related peptide-3 (RFRP-3), NPFF/neuropeptide AF, prolactin-releasing peptides, kisspeptins, and 26/43RFa [6768]. While each family has its own cognate receptor (respectively, NPFF1R, NPFF2R, GPR10, KISS1R, and GPR103), there is increasing evidence for crosstalk signaling amongst the RFamide peptides [171969]. In relation to kisspeptin, recent studies expressing the different RFamide receptors in cell lines have shown that kisspeptin can bind to and activate NPFF1R and NPFFR2 with high affinity [171819]. As NPFFRs are widely expressed in the brain [7071727374], and in several regions where kisspeptin fibers are found, it is possible that kisspeptin signaling in some neurons is mediated by NPFFRs.
One of the first neuroendocrine observations with kisspeptin was that intravenous administration stimulated oxytocin secretion in rats [12]. This has been followed up by Scott and Brown [7576] who demonstrated that intravenous kisspeptin, estimated to achieve plasma concentrations of 1 µM, increased the firing rate of oxytocin neurons in the SON of the rat. However, intracerebroventricular kisspeptin, estimated to achieve a cerebrospinal fluid concentration of >6 µM, did not alter oxytocin firing and the intravenous effects of kisspeptin were abolished by peripheral capsaicin administration. Together, these data indicated that kisspeptin acts peripherally, requiring the vagus nerve, to stimulate oxytocin neurons in female rats [7576].
Remarkably, the effects of kisspeptin on oxytocin neurons appear to change over the course of late-pregnancy and lactation as, at these times, intracerebroventricular kisspeptin is now able to activate oxytocin neuron firing (Fig. 1B) suggesting the appearance of central sites of kisspeptin action within the oxytocin neuronal network [76]. The pathway through which kisspeptin activates oxytocin neurons at these times is not known. There is no evidence for expression of Kiss1r in oxytocin neurons of the SON in rats or mice [1415] although recent data suggest that paraventricular nucleus of hypothalamus (PVN) oxytocin neurons may do so [16]. Notably, NPFF1R is also abundant in the PVN [7273].
There is also evidence that kisspeptin may modulate the activity of magnocellular vasopressin neurons as intracerebroventricular kisspeptin has been found to increase plasma vasopressin concentrations in male rats [77]. Intravenous kisspeptin has also been found to evoke a brief increase in the firing rate of a subpopulation of vasopressin neurons in SON of female rats [75]. As for oxytocin, the mechanism of kisspeptin action is unknown although kisspeptin-10 was recently shown to increase the frequency of miniature excitatory postsynaptic current in mostly vasopressinergic SON neurons in brain slices from male rats [78]. This study suggested that kisspeptin is acting somewhere within the brain slice preparation to modify glutamatergic input to vasopressin neurons. As another possibility, a further recent study has indicated that arcuate neurons co-expressing neurokinin B and kisspeptin may project directly to SON vasopressin neurons [79]. It remains however that very few kisspeptin-immunoreactive fibers have been detected within the SON of the mouse or rat [105979].
Together these studies provide good evidence that kisspeptin is able to modulate that activity of both vasopressin and oxytocin magnocellular neurons although their physiological roles remain unclear. Kisspeptin may act indirectly on these cell types and the precise receptors, sites, and mechanisms of action are unknown.
The ARN contains neural circuits responsible for a wide variety of neuroendocrine and other homeostatic functions. Kisspeptin fibers originating from both the preoptic and ARN kisspeptin neurons [6364], are found in very high density throughout the ARN suggesting possible roles for kisspeptin in modulating many functions of this brain region.
To date, two studies in mice have examined the effects of kisspeptin on neuronal activity in the ARN with both finding that relatively high concentrations of kisspeptin (>100 nM) can excite or inhibit the firing of subpopulations of ARN neurons [2080]. Fu and van den Pol [80] reported that kisspeptin excited pro-opiomelanocortin (POMC) neurons directly by activating non-selective cation channels and a calcium/sodium exchanger. In contrast, those authors found kisspeptin to inhibit neuropeptide Y (NPY) neurons indirectly by activating GABAergic inputs to these cells. As the effects of kisspeptin on POMC neurons were attenuated by pre-treatment with peptide 234, an early KISS1R antagonist, it was suggested that KISS1R mediated the effects of kisspeptin on POMC cells [80]. The receptors involved in the kisspeptin modulation of GABA neurons innervating NPY neurons are not known. It remains possible that kisspeptin acts directly upon NPY neurons as mouse fluorescence-activated cell-sorted green fluorescence protein (GFP)-NPY neurons were reported to express Kiss1r mRNA [81] and kisspeptin fibers are thought to make appositions with NPY neurons in the sheep [82]. It is also noteworthy that intra-cerebroventricular kisspeptin can modulate both Npy and Pomc gene expression [82].
Despite uncertainties regarding the mechanisms of kisspeptin action, it seems clear that kisspeptin is able to modulate the activity of POMC and NPY neurons in the arcuate nucleus of hypothalamus. This has raised the possibility that kisspeptin may be involved in the central control of body weight. To date, it has been shown that intracerebroventricular kisspeptin can dose-dependently inhibit the feeding response to an overnight fast [83] and that female Kiss1r-null mice exhibit elevated body weight associated with impaired glucose tolerance and reduced feeding [84]. However, it is important in these types of experiments to distinguish the direct effects of kisspeptin from confounding indirect effects of kisspeptin manipulations on circulating gonadotropin and gonadal steroid hormone levels. For example, Leon and coworkers [49] recently reported that Kiss1r-null mice genetically-engineered to maintain normal gonadotropin and gonadal hormone levels have normal body weight indicating that kisspeptin signaling is not directly involved in metabolic control.
The second electrophysiological study of kisspeptin actions in the ARN demonstrated that, unexpectedly, kisspeptin was equally effective at modulating ARN neuron firing in wild-type and Kiss1r-null mice [20]. Approximately one-third of all ARN neurons were directly inhibited or excited by kisspeptin in Kiss1r-null mice with the effects of kisspeptin being transient, repeatable and requiring relatively high concentrations (>100 nM) of kisspeptin (Fig. 1C); very different to the characteristics of kisspeptin action on GnRH neurons. This was the first clear indication that kisspeptin could signal independently of KISS1R in the brain. That study went on to show that the effects of RFRP-3, the NPFFR agonist, were very similar if not identical to those of kisspeptin on individual ARN cells (Fig. 1C) as well as the ARN cell population in general [20]. The direct excitatory actions of kisspeptin and RFRP-3 on ARN neurons appeared to result from opening non-selective cation channels whereas the direct inhibitory actions involved opening potassium channels [20]. Alongside evidence for the expression of both NPFFRs in the ARN of mice [85], Liu and Herbison [20] suggested that both the inhibitory and excitatory of kisspeptin in the ARN were mediated by NPFFRs. However, as these studies were not undertaken on neurochemically-identified ARN neurons, it remains possible that kisspeptin actions on POMC neurons are mediated by KISS1R as suggested by Fu and van den Pol [80]. Nevertheless, the vast majority of kisspeptin signaling in the ARN is independent of KISS1R [20]. From a functional perspective, this will endow specific ARN neuron subpopulations with the ability to respond in the same way to both kisspeptin and RFRP-3 inputs.
Although there are no electrophysiological data, It is relevant to note the growing evidence that kisspeptin may modulate the activity of the tuberoinfundibular dopaminergic (TIDA) neurons to regulate prolactin secretion. The intracerebroventricular administration of kisspeptin was found to reduce dopaminergic activity in the median eminence and increase prolactin secretion in rats [8687]. Further, kisspeptin terminals are thought to synapse on TIDA neurons [828788] and kisspeptin inhibits proto-oncogene protein c-fos-related antigen expression in the TIDA neurons [87]. Interestingly, these effects of kisspeptin are larger in females and depend on estrogen [86]. Such data have led to the hypothesis that kisspeptin suppresses the activity of TIDA neurons to elevate prolactin secretion. Although direct recordings of TIDA neurons have not been undertaken, this hypothesis is compatible with the direct inhibitory actions of kisspeptin on unidentified ARN neurons [20]. Interestingly, there is a possibility that prolactin itself feeds back onto the ARN kisspeptin neurons as chronic administration of prolactin increases the expression of pSTAT5 in ARN kisspeptin neurons [8990].
The first electrophysiological investigations into the effects of kisspeptin on neuronal excitability were undertaken by Arai and colleagues [91] working in the hippocampus of the rat. In that study they showed that kisspeptin increased the amplitude of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated postsynaptic currents in granule cells (Fig. 1D); likely reflecting changes in the abundance or kinetics of AMPA receptors expressed by these cells. These effects were unlike those observed with GnRH neurons as they were transient, repeatable, and required high (>200 nM) concentrations of kisspeptin. Kisspeptin was not found to have any other effects upon granule cells, or actions on any other hippocampal cell type [91]. The receptor underlying these actions has not been established with certainty but Kiss1r mRNA is reported within the hippocampus of rats and mice [1314919293] although, curiously, this was not observed in the most recent profiling of Kiss1r mRNA in the rat [16]. As NPFF1R is also found throughout the hippocampus [7374] it cannot as yet be excluded that kisspeptin signals through NPFFRs to regulate hippocampal function.
It remains unclear whether the kisspeptin peptide itself is found in the hippocampus. Immunohistochemical studies have consistently failed to detect kisspeptin-immunoreactive fibers within the hippocampus of rats or mice [101159]. Nevertheless, Kiss1 mRNA has been reported in the hippocampus, although its expression level is about 50-fold lower than that of the hypothalamus [13919293]. This suggests that low levels of kisspeptin peptide may be produced within the hippocampus itself and this may be elevated by seizure activity [92]. Interestingly, high concentrations of kisspeptin (10 µM) increase brainderived neurotrophic factor (BDNF) mRNA in organotypic hippocampal slice cultures and BDNF is reported to enhance synaptic transmission in dentate gyrus [94].
The functional role of kisspeptin signaling in the hippocampus is unknown. It was recently shown that kisspeptin-13 promoted memory formation and retention in male mice [95]. It is notable that this required very high intracerebroventricular concentrations of kisspeptin (~15 nmol/mouse) compared to the intracerebroventricular levels needed to stimulate GnRH secretion (<0.01 nmol/mouse) [9697]. Furthermore, kisspeptin's actions on memory were blocked by a GnRH receptor antagonist [95] indicating that indirect actions of kisspeptin involving the activation of secretion of gonadotropins and gonadal steroid hormone were critical. Circulating levels of luteinizing hormone (LH) and estrogen have long been known to modulate hippocampal function [9899]. Nevertheless, it was also reported that the bilateral injection of ~3 nmol kisspeptin-13 directly into the hippocampus improved memory suggesting a local action [95]. It would appear that kisspeptin interacts with many different neurotransmitter systems in the hippocampus as the effects of intracerebroventricular kisspeptin-13 on passive avoidance learning in mice are dependent upon adrenergic, serotoninergic, acetylcholinergic, dopaminergic, GABAergic, and nitric oxide signaling [100]. Further the administration of a KISS1R antagonist was not found to have any effects on hippocampal function [95]. These features indicate that kisspeptin acts as a neuropeptide neuromodulator in the hippocampus.
The medial amygdala is another brain region where kisspeptin fibers are detected alongside a small number of kisspeptin neurons in the mouse and rat [62101102103]. At present, the role of kisspeptin fibers in the amygdala is unknown and no electrophysiological studies have examined the actions of kisspeptin in this brain region. It is notable that the recent distribution studies have not detected KISS1R within the medial amygdala of mice [1516] although it is found in rats [14]. Recently, Comninos and colleagues [104] used manganese-enhanced magnetic resonance imaging in rats to show that peripheral administration of kisspeptin-54 decreased the activity of the medial amygdala by ~20%. In subsequent studies those authors found that administration of high concentrations (1 nmol) of kisspeptin directly into the medial amygdala resulted in an increase in LH secretion while treatment with the kisspeptin antagonist peptide-234 reduced LH pulsatility [104]. These interesting observations suggest that kisspeptin signaling within the medial amygdala can in some way suppress GnRH neuron functioning and support prior studies implicating this brain region in fertility control [105106107].
This review highlights increasing evidence for functionally-relevant kisspeptin signaling within multiple different brain regions. While a special role for kisspeptin in the regulation of GnRH neurons exists, it is apparent that many other neuronal networks unrelated to fertility control also utilize kisspeptin as a neuropeptide to modulate activity. From an evaluation of these different roles of kisspeptin in the brain, two major themes are evident.
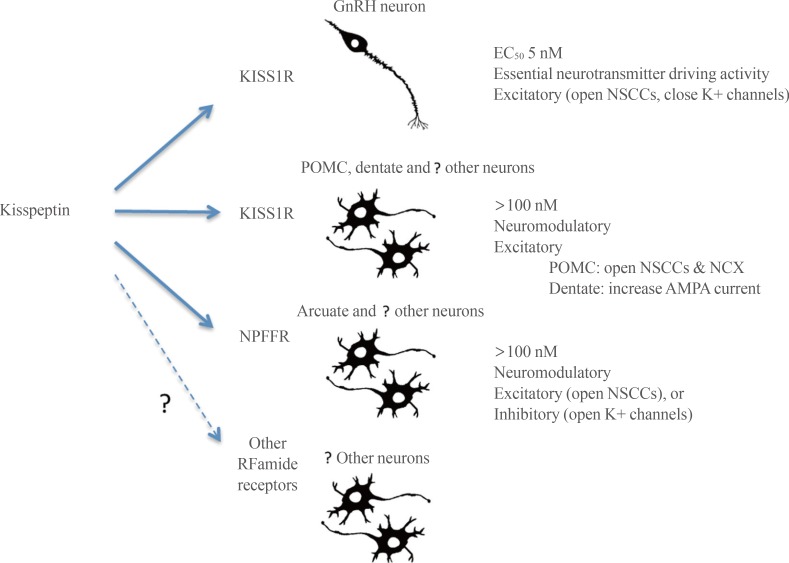
First, kisspeptin-KISS1R signaling at the GnRH neuron appears to be fundamentally different to kisspeptin neuropeptidergic transmission occurring elsewhere in the brain (Fig. 2). At the GnRH neuron, kisspeptin activation of KISS1R is remarkably potent with an EC50 of 3 to 5 nM [3738] and an ability to drive GnRH neuron firing that is more robust than that observed for glutamate [31]. This is unusual for a neuropeptide that would typically be expected to exert a more moderate neuromodulatory role acting in the >100 nM range. For example a range of other neuropeptides require 100 to 1,000 nM concentrations to be able to modulate the firing of GnRH neurons [108109]. Given the highly conserved role of kisspeptin in regulating GnRH neurons in vertebrate species [110], it is possible that this unusually potent form of neuropeptidergic control of the GnRH neurons is evolutionarily ancient and has been maintained due to the absolute necessity of reproduction for species propagation.
Elsewhere in the brain, high concentrations of kisspeptin are required to modulate neuronal activity or functioning. For example, even in brain areas where KISS1R may be expressed, electrophysiological studies require 100 to 1,000 nM levels of kisspeptin to exert significant effects and intracerebroventricular concentrations need to be 1,000-fold greater (see above). This suggests that kisspeptin acts as a neuropeptide with a typical neuromodulatory mode of action in the wider brain (Fig. 2). Indeed, this principal seems likely to be applicable to kisspeptin signaling throughout the body [89] and is compatible with the absence of any major phenotype beyond defective GnRH neuron functioning in humans or animals with kisspeptin-related mutations [13549].
The second theme is that kisspeptin is able to act through KISS1R as well as other receptors, primarily NPFFRs, to modulate neuronal activity. As noted in this review, there are several brain locations where a mismatch occurs between the presence of kisspeptin fibers and Kiss1r mRNA expression. In some cases, kisspeptin fiber location better matches the expression of NPFFRs. Further, electrophysiological studies clearly show that kisspeptin, acting at neuromodulatory concentrations (100 to 400 nM), can have the same effects as NPFFR agonists and also regulate neuronal firing in the absence of KISS1R. This in itself is not unusual for neuropeptidergic signaling in the brain (and periphery) where a neuropeptide, whilst having highest affinity for one subtype, can nevertheless activate multiple receptors within its family. Clearly much further investigation is required in this area. The further development of NPFFR-null mice [111], specific NPFFR agonists and antagonists [52], and the generation of reliable antisera to KISS1R and the NPFFRs will be critical for investigating RFamide neuropeptide cross-talk signaling in the brain.
We conclude by speculating that kisspeptin will be found to act as a neuromodulatory neuropeptide within multiple different neuronal networks in the brain. In common with other neuropeptides, these effects will likely be mediated by multiple neuropeptide receptors and not found to be essential for the functional integrity of the neuronal network they signal within. This is in marked contrast to kisspeptin-KISS1R signaling at the GnRH neuron that is an unusually potent and irreplaceable form of neuropeptidergic neurotransmission.
ACKNOWLEDGMENTS
The authors thank Associate Professor Greg Anderson for commenting on an earlier version of the manuscript. The Health Research Council of New Zealand is thanked for financial support.
References
1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003; 100:10972–10976. PMID: 12944565.

2. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003; 312:1357–1363. PMID: 14652023.

3. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003; 349:1614–1627. PMID: 14573733.

4. Clarke H, Dhillo WS, Jayasena CN. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol Metab (Seoul). 2015; 30:124–141. PMID: 26194072.

5. Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013; 4:2492. PMID: 24051579.

6. Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008; 70:213–238. PMID: 17988212.

7. Roa J, Navarro VM, Tena-Sempere M. Kisspeptins in reproductive biology: consensus knowledge and recent developments. Biol Reprod. 2011; 85:650–660. PMID: 21677307.
8. Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology. 2015; 156:1218–1227. PMID: 25590245.

9. Hussain MA, Song WJ, Wolfe A. There is kisspeptin: and then there is kisspeptin. Trends Endocrinol Metab. 2015; 26:564–572. PMID: 26412157.
10. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009; 21:673–682. PMID: 19515163.

11. Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009; 30:26–33. PMID: 18840491.

12. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001; 276:34631–34636. PMID: 11457843.

13. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001; 276:28969–28975. PMID: 11387329.

14. Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999; 446:103–107. PMID: 10100623.

15. Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010; 151:312–321. PMID: 19966188.

16. Higo S, Honda S, Iijima N, Ozawa H. Mapping of kisspeptin receptor mRNA in the whole rat brain and its co-localization with oxytocin in the paraventricular nucleus. J Neuroendocrinol. 2016; 28(4):

17. Elhabazi K, Humbert JP, Bertin I, Schmitt M, Bihel F, Bourguignon JJ, et al. Endogenous mammalian RF-amide peptides, including PrRP, kisspeptin and 26RFa, modulate nociception and morphine analgesia via NPFF receptors. Neuropharmacology. 2013; 75:164–171. PMID: 23911743.

18. Lyubimov Y, Engstrom M, Wurster S, Savola JM, Korpi ER, Panula P. Human kisspeptins activate neuropeptide FF2 receptor. Neuroscience. 2010; 170:117–122. PMID: 20600636.

19. Oishi S, Misu R, Tomita K, Setsuda S, Masuda R, Ohno H, et al. Activation of neuropeptide FF receptors by kisspeptin receptor ligands. ACS Med Chem Lett. 2010; 2:53–57. PMID: 24900254.

20. Liu X, Herbison A. Kisspeptin regulation of arcuate neuron excitability in kisspeptin receptor knockout mice. Endocrinology. 2015; 156:1815–1827. PMID: 25756309.

21. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996; 88:1731–1737. PMID: 8944003.

22. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001; 411:613–617. PMID: 11385580.

23. Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004; 117(Pt 8):1319–1328. PMID: 15020672.

24. Stafford LJ, Xia C, Ma W, Cai Y, Liu M. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002; 62:5399–5404. PMID: 12359743.
25. Kirby HR, Maguire JJ, Colledge WH, Davenport AP. International union of basic and clinical pharmacology. LXXVII. Kisspeptin receptor nomenclature, distribution, and function. Pharmacol Rev. 2010; 62:565–578. PMID: 21079036.

26. Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: a historical perspective and suggested nomenclature. Peptides. 2009; 30:4–9. PMID: 18644415.
27. Kanda S, Oka Y. Evolutionary insights into the steroid sensitive kiss1 and kiss2 neurons in the vertebrate brain. Front Endocrinol (Lausanne). 2012; 3:28. PMID: 22654859.

28. Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, Osugi T, et al. Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology. 2009; 150:2837–2846. PMID: 19164475.

29. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005; 25:11349–11356. PMID: 16339030.

30. Alreja M. Electrophysiology of kisspeptin neurons. Adv Exp Med Biol. 2013; 784:349–362. PMID: 23550014.

31. Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2015; 36:15–27. PMID: 24907402.

32. Ronnekleiv OK, Kelly MJ. Kisspeptin excitation of GnRH neurons. Adv Exp Med Biol. 2013; 784:113–131. PMID: 23550004.
33. Choe HK, Chun SK, Kim J, Kim D, Kim HD, Kim K. Real-time GnRH gene transcription in GnRH promoter-driven luciferase-expressing transgenic mice: effect of kisspeptin. Neuroendocrinology. 2015; 102:194–199. PMID: 25571901.

34. Liu X, Porteous R, d'Anglemont de Tassigny X, Colledge WH, Millar R, et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011; 31:2421–2430. PMID: 21325509.

35. Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008; 28:8003–8013. PMID: 18685025.

36. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008; 149:4605–4614. PMID: 18483150.

37. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008; 149:1979–1986. PMID: 18162521.

38. Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008; 28:4423–4434. PMID: 18434521.

39. Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013; 33:9394–9401. PMID: 23719807.

40. Zhang XB, Spergel DJ. Kisspeptin inhibits high-voltage activated Ca2+ channels in GnRH neurons via multiple Ca2+ influx and release pathways. Neuroendocrinology. 2012; 96:68–80. PMID: 22343183.
41. Zhang C, Bosch MA, Ronnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology. 2013; 154:2772–2783. PMID: 23744639.

42. Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011; 152:3832–3841. PMID: 21791557.

43. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008; 149:3926–3932. PMID: 18450966.
44. Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015; 156:231–241. PMID: 25314270.

45. Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011; 152:1001–1012. PMID: 21239443.

46. Uenoyama Y, Inoue N, Pheng V, Homma T, Takase K, Yamada S, et al. Ultrastructural evidence of kisspeptin-gonadotrophin-releasing hormone (GnRH) interaction in the median eminence of female rats: implication of axo-axonal regulation of GnRH release. J Neuroendocrinol. 2011; 23:863–870. PMID: 21815953.

47. Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, d'Anglemont de Tassigny X, et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci. 2012; 32:932–945. PMID: 22262891.

48. Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014; 28:225–238. PMID: 24422632.

49. Leon S, Barroso A, Vazquez MJ, Garcia-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, et al. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci Rep. 2016; 6:19206. PMID: 26755241.

50. Simonin F, Schmitt M, Laulin JP, Laboureyras E, Jhamandas JH, MacTavish D, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci U S A. 2006; 103:466–471. PMID: 16407169.

51. Liu X, Herbison AE. RF9 excitation of GnRH neurons is dependent upon Kiss1r in the adult male and female mouse. Endocrinology. 2014; 155:4915–4924. PMID: 25322463.

52. Kim JS, Brownjohn PW, Dyer BS, Beltramo M, Walker CS, Hay DL, et al. Anxiogenic and stressor effects of the hypothalamic neuropeptide RFRP-3 are overcome by the NPFFR antagonist GJ14. Endocrinology. 2015; 156:4152–4162. PMID: 26259035.

53. Min L, Leon S, Li H, Pinilla L, Carroll RS, Tena-Sempere M, et al. RF9 acts as a KISS1R agonist in vivo and in vitro. Endocrinology. 2015; 156:4639–4648. PMID: 26418326.

54. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014; 34:15060–15069. PMID: 25378170.

55. Caraty A, Blomenrohr M, Vogel GM, Lomet D, Briant C, Beltramo M. RF9 powerfully stimulates gonadotrophin secretion in the ewe: evidence for a seasonal threshold of sensitivity. J Neuroendocrinol. 2012; 24:725–736. PMID: 22283564.

56. Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, et al. Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: physiological and pharmacological implications. Endocrinology. 2010; 151:1902–1913. PMID: 20160130.

57. Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, et al. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology. 2012; 153:3770–3779. PMID: 22691552.

58. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013; 784:27–62. PMID: 23550001.

59. Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, et al. Mapping of kisspeptin fibres in the brain of the pro-oestrous rat. J Neuroendocrinol. 2010; 22:1101–1112. PMID: 20673302.

60. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010; 166:680–697. PMID: 20038444.

61. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011; 23:52–64. PMID: 21029216.

62. Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, et al. Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J. 2012; 59:161–171. PMID: 22240892.

63. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011; 152:2387–2399. PMID: 21486932.

64. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015; 156:2582–2594. PMID: 25856430.

65. Franceschini I, Yeo SH, Beltramo M, Desroziers E, Okamura H, Herbison AE, et al. Immunohistochemical evidence for the presence of various kisspeptin isoforms in the mammalian brain. J Neuroendocrinol. 2013; 25:839–851. PMID: 23822722.

66. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005; 102:2129–2134. PMID: 15684075.

67. Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006; 27:1073–1086. PMID: 16500002.

68. Dockray GJ. The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp Physiol. 2004; 89:229–235. PMID: 15123557.

69. Ma L, MacTavish D, Simonin F, Bourguignon JJ, Watanabe T, Jhamandas JH. Prolactin-releasing peptide effects in the rat brain are mediated through the neuropeptide FF receptor. Eur J Neurosci. 2009; 30:1585–1593. PMID: 19821834.

70. Parker RM, Copeland NG, Eyre HJ, Liu M, Gilbert DJ, Crawford J, et al. Molecular cloning and characterisation of GPR74 a novel G-protein coupled receptor closest related to the Y-receptor family. Brain Res Mol Brain Res. 2000; 77:199–208. PMID: 10837915.

71. Bonini JA, Jones KA, Adham N, Forray C, Artymyshyn R, Durkin MM, et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J Biol Chem. 2000; 275:39324–39331. PMID: 11024015.

72. Gouarderes C, Puget A, Zajac JM. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: a comparative autoradiographic study. Synapse. 2004; 51:249–269. PMID: 14696013.
73. Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001; 276:36961–36969. PMID: 11481330.

74. Goncharuk V, Zeng Z, Wang R, MacTavish D, Jhamandas JH. Distribution of the neuropeptide FF1 receptor (hFF1) in the human hypothalamus and surrounding basal forebrain structures: immunohistochemical study. J Comp Neurol. 2004; 474:487–503. PMID: 15174068.

75. Scott V, Brown CH. Kisspeptin activation of supraoptic nucleus neurons in vivo. Endocrinology. 2011; 152:3862–3870. PMID: 21810945.

76. Scott V, Brown CH. Beyond the GnRH axis: kisspeptin regulation of the oxytocin system in pregnancy and lactation. Adv Exp Med Biol. 2013; 784:201–218. PMID: 23550008.

77. Han X, Yan M, An XF, He M, Yu JY. Central administration of kisspeptin-10 inhibits natriuresis and diuresis induced by blood volume expansion in anesthetized male rats. Acta Pharmacol Sin. 2010; 31:145–149. PMID: 20023694.

78. Yokoyama T, Minami K, Terawaki K, Miyano K, Ogata J, Maruyama T, et al. Kisspeptin-10 potentiates miniature excitatory postsynaptic currents in the rat supraoptic nucleus. Brain Res. 2014; 1583:45–54. PMID: 25130664.

79. Pineda Reyes R, Sabatier N, Ludwig M, Millar RP, Leng G. A direct neurokinin B projection from the arcuate nucleus regulates magnocellular vasopressin cells of the supraoptic nucleus. J Neuroendocrinol. 2016; 28(4):

80. Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010; 30:10205–10219. PMID: 20668204.

81. Kim GL, Dhillon SS, Belsham DD. Kisspeptin directly regulates neuropeptide Y synthesis and secretion via the ERK1/2 and p38 mitogen-activated protein kinase signaling pathways in NPY-secreting hypothalamic neurons. Endocrinology. 2010; 151:5038–5047. PMID: 20685868.

82. Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010; 151:2233–2243. PMID: 20207832.

83. Stengel A, Wang L, Goebel-Stengel M, Tache Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport. 2011; 22:253–257. PMID: 21386700.

84. Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014; 124:3075–3079. PMID: 24937427.

85. Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol. 2013; 25:876–886. PMID: 23927071.

86. Ribeiro AB, Leite CM, Kalil B, Franci CR, Anselmo-Franci JA, Szawka RE. Kisspeptin regulates tuberoinfundibular dopaminergic neurones and prolactin secretion in an oestradiol-dependent manner in male and female rats. J Neuroendocrinol. 2015; 27:88–99. PMID: 25453900.

87. Szawka RE, Ribeiro AB, Leite CM, Helena CV, Franci CR, Anderson GM, et al. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology. 2010; 151:3247–3257. PMID: 20410200.

88. Sawai N, Iijima N, Takumi K, Matsumoto K, Ozawa H. Immunofluorescent histochemical and ultrastructural studies on the innervation of kisspeptin/neurokinin B neurons to tuberoinfundibular dopaminergic neurons in the arcuate nucleus of rats. Neurosci Res. 2012; 74:10–16. PMID: 22691459.

89. Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014; 155:1010–1020. PMID: 24456164.

90. Brown RS, Herbison AE, Grattan DR. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. J Neuroendocrinol. 2014; 26:898–908. PMID: 25207795.

91. Arai AC, Xia YF, Suzuki E, Kessler M, Civelli O, Nothacker HP. Cancer metastasis-suppressing peptide metastin upregulates excitatory synaptic transmission in hippocampal dentate granule cells. J Neurophysiol. 2005; 94:3648–3652. PMID: 16222076.

92. Arai AC, Orwig N. Factors that regulate KiSS1 gene expression in the hippocampus. Brain Res. 2008; 1243:10–18. PMID: 18834866.

93. Arai AC. The role of kisspeptin and GPR54 in the hippocampus. Peptides. 2009; 30:16–25. PMID: 18765263.

94. Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998; 79:496–499. PMID: 9425220.

95. Jiang JH, He Z, Peng YL, Jin WD, Wang Z, Han RW, et al. Kisspeptin-13 enhances memory and mitigates memory impairment induced by Aβ1-42 in mice novel object and object location recognition tasks. Neurobiol Learn Mem. 2015; 123:187–195. PMID: 26103138.

96. Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005; 146:156–163. PMID: 15375028.

97. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004; 145:4073–4077. PMID: 15217982.

98. Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995; 29:42–58. PMID: 7782062.

99. Luine V. Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents. J Steroid Biochem Mol Biol. 2016; 160:189–195. PMID: 26241030.

100. Telegdy G, Adamik A. The action of kisspeptin-13 on passive avoidance learning in mice. Involvement of transmitters. Behav Brain Res. 2013; 243:300–305. PMID: 23348107.

101. Cao J, Patisaul HB. Sex-specific expression of estrogen receptors α and β and Kiss1 in the postnatal rat amygdala. J Comp Neurol. 2013; 521:465–478. PMID: 22791648.

102. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011; 152:2020–2030. PMID: 21363930.

103. Di Giorgio NP, Semaan SJ, Kim J, Lopez PV, Bettler B, Libertun C, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014; 155:1033–1044. PMID: 24424047.

104. Comninos AN, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, Hu M, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016; 221:2035–2047. PMID: 25758403.

105. Beltramino C, Taleisnik S. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res. 1978; 144:95–107. PMID: 565243.

106. Velasco ME, Taleisnik S. Effects of the interruption of amygdaloid and hippocampal afferents to the medial hypothalmus on gonadotrophin release. J Endocrinol. 1971; 51:41–55. PMID: 5166464.
107. Bagga N, Chhina GS, Kumar VM, Singh B. Cholinergic activation of medial preoptic area by amygdala for ovulation in rat. Physiol Behav. 1984; 32:45–48. PMID: 6718533.

108. Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012; 153:5587–5599. PMID: 22948210.

109. Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005; 132:703–712. PMID: 15837132.

110. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009; 30:713–743. PMID: 19770291.

111. Leon S, Garcia-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology. 2014; 155:2953–2965. PMID: 24823392.
Fig. 1
Kisspeptin actions on the excitability of different central nervous system (CNS) neurons. (A) Voltage recording of gonadotropin-releasing hormone (GnRH) neuron action potential firing from a female green fluorescent protein-GnRH mouse showing the typical long-lasting excitation evoked by a short 2-minute (grey bar) application of 10 nM kisspeptin. (B) Ratemeter histograms of action potential firing in two oxytocin neurons from a urethane-anaesthetized day 18 pregnant rat showing the effects of intracerebroventricular injection (ICV) and intravenous injection (IV) of kisspeptin, respectively (recordings kindly provided by Drs V. Scott, A.J. Seymour, and C.H. Brown, University of Otago, Dunedin, New Zealand). (C) Voltage recording of ARC neuron action potential firing from a Kiss1r-null female mouse showing short-lasting excitatory responses to 400 nM kisspeptin and RFRP-3. Adapted from Liu et al. [20], with permission from Endocrine Society. (D) Whole cell current recordings from hippocampal dentate granule neurons in rats showing that 600 nM kisspeptin increases the amplitude of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated excitatory postsynaptic currents (EPSC). Left, histogram of mean response. Right, example of EPSCs during control and after 600 nM kisspeptin (recordings kindly provided by Prof. Amy Arai, Department of Pharmacology, Southern Illinois University School of Medicine, Springfield, IL, USA).
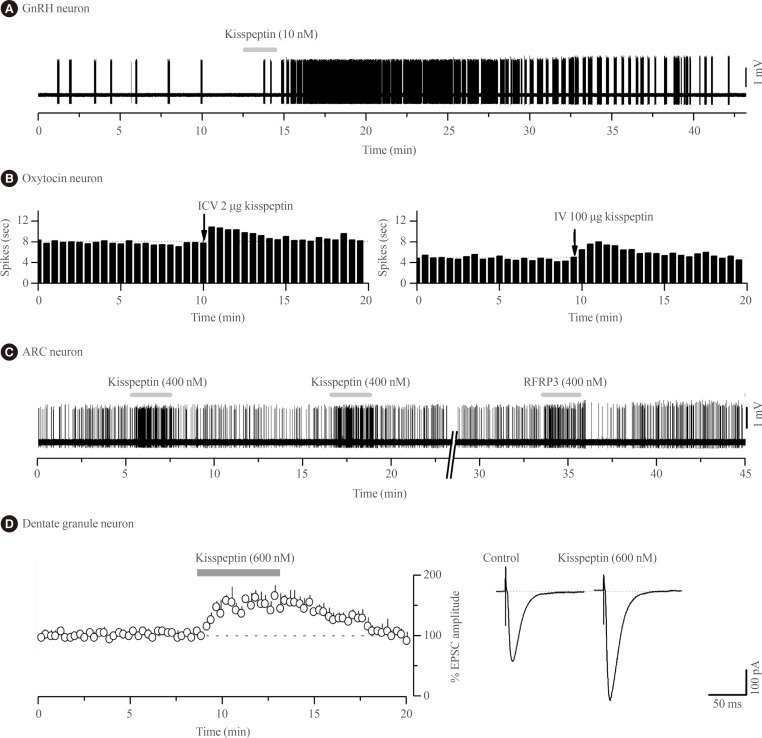
Fig. 2
Schematic diagram showing possible signaling pathways for kisspeptin in the central nervous system (CNS). The distinction is made between signaling through kisspeptin receptor (KISS1R), neuropeptide FF receptor (NPFFR) and possibly even other RFamide receptors. Signaling through KISS1R may be either an essential synaptic driver (gonadotropin-releasing hormone [GnRH] neurons) or neuromodulatory (proposed for other CNS neurons), whereas signaling through NPFFRs is suggested to be neuromodulatory throughout the CNS. The primary ion channels modulated by kisspeptin are noted for each mode of signaling. NSCC, non-selective cation ion channels; POMC, pro-opiomelanocortin; NCX, sodium-calcium exchanger; AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download