Abstract
Allergic reaction to insulin is uncommon since the introduction of human recombinant insulin preparations and is more rare in pregnant than non-pregnant females due to altered immune reaction during pregnancy. Herein, we report two cases of allergic reaction to insulin in gestational diabetes that were successfully managed. One case was a 33-year-old female using isophane-neutral protamine Hagedorn human insulin and insulin lispro. She experienced dyspnea, cough, urticaria and itching sensation at the sites of insulin injection immediately after insulin administration. We discontinued insulin therapy and started oral hypoglycemic agents with metformin and glibenclamide. The other case was a 32-year-old female using insulin lispro and insulin detemer. She experienced pruritus and burning sensation and multiple nodules at the sites of insulin injection. We changed the insulin from insulin lispro to insulin aspart. Assessments including immunoglobulin E (IgE), IgG, eosinophil, insulin antibody level and skin biopsy were performed. In the two cases, the symptoms were resolved after changing the insulin to oral agents or other insulin preparations. We report two cases of allergic reaction to human insulin in gestational diabetes due to its rarity.
The incidence of allergic reaction to insulin is 0.1% to 3% of diabetic patients treated with insulin [1]. Allergy to insulin in gestational diabetes is extremely rare due to the altered immune reaction during pregnancy [2]. Allergic reactions range from local injection site reactions to severe generalized anaphylactic, life-threatening reactions. Herein, we report two cases of allergic reaction to insulin in gestational diabetes patients that were successfully managed. To our knowledge, this is the first report of allergic reaction to insulin in gestational diabetes in Korea.
A 33-year-old female (weight 82.4 kg, body mass index [BMI] 33 kg/m2) was admitted to our hospital because of dyspnea, cough, urticaria, and itching sensation at the site of insulin injection immediately after insulin administration at 36+5 weeks of pregnancy. On admission, the symptoms of dyspnea and cough had disappeared. The patient had gestational diabetes and was treated with neutral protamine Hagedorn human insulin and insulin lispro for 2 months. She had no underlying disease and no history of allergy. On admission, her blood pressure was 100/60 mm Hg, heart rate was 84 beats per minute, respiratory rate was 16 breaths per minute and body temperature was 36.4℃. An asymptomatic grayish and reddish hyperpigmented patches were observed on both proximal arms (Fig. 1). Laboratory findings showed a white blood cell (WBC) count of 15,030 cells/µL (eosinophil count 620/µL), immunoglobulin E (IgE) 171.4 IU/mL (range, 0 to 100), IgG 714.0 mg/dL (range, 700 to 1,600), insulin antibody 34.4% (range, 0 to 8.2), and hemoglobin A1c (HbA1c) was 6.2%. Skin biopsy of the patches on the arms revealed perivascular lymphohistiocytic and eosinophilic infiltration in the deep dermis and subcutis (Fig. 2). Insulin therapy was discontinued and oral hypoglycemic agents (metformin and glibenclamide) were started. The blood sugar level was well maintained by oral agents only and the patient was discharged on day 4 of hospitalization with clinical improvement. After 1 week, eosinophil count, IgE and insulin antibody levels were measured and were decreased (420/µL, 170.0 IU/mL, and 7.0%, respectively). The patient gave birth via Cesarean section (C-section) at 38 weeks of pregnancy and the baby weighed 3.2 kg.
A 32-year-old female (weight 73.8 kg, BMI 32.36 kg/m2) with no underlying disease was diagnosed with gestational diabetes at 27+4 weeks of pregnancy. She was pregnant previously without gestational diabetes. Her HbA1c was 6.2% and she was started on a treatment with insulin lispro and insulin detemer. The blood sugar level was well controlled and she was discharged with insulin on day 12 of hospitalization. One week after discharge, she visited the outpatient clinic for a follow-up and presented with multiple erythematous nodules on both thighs where the insulin was injected (Fig. 3). She complained of pruritus and burning sensation at the sites, but there was no tenderness. The symptoms were aggravated mostly after injection of insulin lispro. We suspected insulin hypersensitivity and performed skin biopsy and blood sampling; consequently a topical steroid ointment was prescribed. Upon laboratory analysis, WBC count was 9,500 cells/µL (eosinophil count, 1.8%), IgE 121.3 IU/mL, IgG 781.0 mg/dL and insulin antibody was negative. The skin biopsy revealed spongiosis of epidermis, perivascular mononuclear cell infiltration in the dermis and eosinophilic infiltration in the subcutis (Fig. 4). The skin lesions were aggravated and mitigated during maintenance of initial insulin treatment and the IgE level was increased to 163.4 IU/mL. The treatment was changed from insulin lispro to insulin aspart and insulin detemer was continued. After changing the type of insulin, the skin lesions were resolved. She maintained insulin therapy using insulin aspart and insulin detemer without side effects and gave birth to a 3-kg baby via C-section at 37+3 weeks of pregnancy.
Allergic reaction to insulin has been uncommon since the introduction of human recombinant insulin preparations instead of animal insulin; the frequency ranges from 0.1% to 3% during insulin treatment [1].
The exact prevalence of anaphylaxis and allergy to insulin during pregnancy is unknown, but the condition is very rare during pregnancy due to altered immune reactions [23]. Pregnancy is often considered a period of pure immunosuppression because of the autoimmune disease improvement and the increased susceptibility to influenza infections during pregnancy. During pregnancy, T cells, B cells, and natural killer cells are transcriptionally downregulated by estrogen and progesterone [2].
Multiple insulin components-such as pharmaceutical formula additives (protamine, metacresol, or zinc) and latex-and insulin itself can act as the allergen [1].
The mechanisms of insulin allergy can be of three types: hypersensitivity I, III, and IV. The IgE-mediated type I hypersensitivity is the most common, usually localized at the injection site and rarely causes anaphylaxis [4]. IgG-mediated type III immune complex-type hypersensitivity and immune T cell-mediated delayed-type type IV hypersensitivity occur infrequently [5].
IgE-mediated symptoms occur immediately after insulin administration and biphasically followed by a more sustained induration and generalized reaction 4 to 8 hours later. Type III reactions occur at the injection site as subcutaneous nodules after 2 to 6 hours after insulin administration [4]. Type IV reactions occur usually as a reaction to the retarding agents in insulin preparations [6].
For accurate diagnosis, skin prick test, patch test, intradermal test, specific IgE and occasionally, skin biopsy can be used. However, history taking is the most important [4].
In our two cases, eosinophil, IgE and IgG levels were measured and a skin biopsy performed. The IgE level was elevated, IgG level was normal, eosinophilia was observed and skin biopsy showed eosinophilic infiltration. Our two insulin allergy cases were considered type I hypersensitivity because of the elevated IgE. The first case was a more severe form with accompanying systemic symptoms, such as dyspnea and cough, compared with the second case, that showed localized skin lesions only.
Various treatment options are available for managing allergic reactions to insulin [1]. Antihistamines and corticosteroids can be used for symptom relief. Changing the insulin to different oral hypoglycemic agents or insulin preparations is also important [1]. Additionally, use of insulin as a continuous subcutaneous infusion can be helpful. Subcutaneous insulin desensitization is a specific immunotherapy that increases insulin dose progressively under close observation [14]. In our cases, the symptoms were improved after changing the insulin regimen to another oral agent or preparation due to subtle differences in antigenicity.
In case 1, the patient had only 3 weeks to childbirth and presented with insulin antibodies. Therefore, we changed the insulin to metformin and glibenclamide although the drugs were classified as category B and C in pregnancy, respectively. The American College of Obstetrics and Gynecology and the UK National Institute of Health and Care Excellence recommend that either metformin or glibenclamide can be used to treat gestational diabetes [78].
In case 2, the allergic reaction to insulin occurred when insulin lispro and insulin detemer were used. The symptoms became aggravated after injection of insulin lispro; thus, the insulin lispro was considered the allergen. The patient's laboratory findings showed no insulin antibodies; a previous case where insulin lispro caused localized allergic reactions was reported, but insulin aspart was less immunogenic [9]. Therefore, insulin lispro was changed to aspart, and no allergic reaction occurred after the insulin regimen was changed. Subsequently, the symptoms were resolved.
Insulin lispro has subtle structural differences from human insulin (substitution of proline with lysine in position 28 and lysine with proline in position 29 of the B chain) and includes inactive ingredients such as glycerol, phenol, metacresol, or zinc [410]. Conversely, insulin aspart differs from insulin lispro structurally (a single substitution of proline with aspartic acid in position B28) and in doses of metacresol in preparation (lispro 3.15 mg/dL, aspart 1.72 mg/dL) [10111213], which may have caused a difference in allergic reaction. Case 2 is interesting because allergy to insulin was thought to be caused by insulin lispro, which has a low immunogenic potency and is usually administered to patients who suffer from insulin allergy [14].
In summary, we reported two cases of allergic reaction to human insulin in patients with gestational diabetes. These cases present interesting and unique aspects because insulin allergy in gestational diabetes is extremely infrequent owing to its immunosuppressive state [2]. To our knowledge, less than 10 cases of allergy to insulin in gestational diabetes have been reported globally [1516171819202122] and this is the first report of insulin allergy in gestational diabetes in Korea. Therefore, we report two cases of allergic reaction to insulin in patients with gestational diabetes and a review of the literature.
Figures and Tables
Fig. 1
Grayish and reddish hyperpigmented patches at the site of insulin injection. (A) Right proximal arm. (B) Left proximal arm.
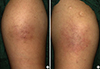
Fig. 2
Histopathology of skin lesions of both proximal arms. Perivascular lymphohistiocytic and eosinophilic infiltration (A) around fat tissue in deep dermis (H&E stain, ×400) and (B) around capillaries in subcutis (H&E stain, ×400) was observed.

Fig. 3
Multiple erythematous nodules at the site of insulin injection. (A) Right thigh. (B) Left thigh.
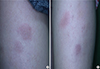
Fig. 4
Histopathology of skin lesions of both thighs. (A) Spongiosis of epidermis (H&E stain, ×400). (B) Perivascular mononuclear cell infiltration around endothelial cells at the dermis level (H&E stain, ×400). (C) Eosinophilic infiltration around capillaries at the subcutis level (H&E stain, ×400).

References
1. Jacquier J, Chik CL, Senior PA. A practical, clinical approach to the assessment and management of suspected insulin allergy. Diabet Med. 2013; 30:977–985.
2. Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res. 2012; 54:254–261.
3. Pali-Scholl I, Motala C, Jensen-Jarolim E. Asthma and allergic diseases in pregnancy a review. World Allergy Organ J. 2009; 2:26–36.
4. Ghazavi MK, Johnston GA. Insulin allergy. Clin Dermatol. 2011; 29:300–305.
5. Hong JH, Lee JH, Shin JH, Kim DP, Ko BS, Kim BJ, et al. Maintenance of insulin therapy by desensitization in insulin allergy patient. Korean Diabetes J. 2008; 32:529–531.
6. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006; 23:204–206.
7. Holt RI, Lambert KD. The use of oral hypoglycaemic agents in pregnancy. Diabet Med. 2014; 31:282–291.
8. Kimber-Trojnar Z, Marciniak B, Patro-Malysza J, Skorzynska-Dziduszko K, Poniedzialek-Czajkowska E, Mierzynski R, et al. Is glyburide safe in pregnancy? Curr Pharm Biotechnol. 2014; 15:100–112.
9. Airaghi L, Lorini M, Tedeschi A. The insulin analog aspart: a safe alternative in insulin allergy. Diabetes Care. 2001; 24:2000.
10. Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care. 2003; 26:2027–2031.
11. Hoffman AG, Schram SE, Ercan-Fang NG, Warshaw EM. Type I allergy to insulin: case report and review of localized and systemic reactions to insulin. Dermatitis. 2008; 19:52–58.
12. Kim D, Baraniuk J. Delayed-type hypersensitivity reaction to the meta-cresol component of insulin. Ann Allergy Asthma Immunol. 2007; 99:194–195.
13. Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Exp Diabetes Res. 2010; 2010:178372.
14. Kumar D. Lispro analog for treatment of generalized allergy to human insulin. Diabetes Care. 1997; 20:1357–1359.
15. Durand-Gonzalez KN, Guillausseau N, Anciaux ML, Hentschel V, Gayno JP. Allergy to insulin in a woman with gestational diabetes mellitus: transient efficiency of continuous subcutaneous insulin lispro infusion. Diabetes Metab. 2003; 29(4 Pt 1):432–434.
16. Balsells MC, Corcoy RM, Lleonart RB, Pujol RV, Mauricio DP, Garcia AP, et al. Primary allergy to human insulin in patient with gestational diabetes. Diabetes Care. 1991; 14:423–424.
17. Gossain VV, Rovner DR, Mohan K. Systemic allergy to human (recombinant DNA) insulin. Ann Allergy. 1985; 55:116–118.
18. Sokup A, Swiatkowski M, Tyloch M, Szymanski W. Insulin Lispro as an alternative for insulin Humulin U in the treatment of an obese gestational diabetic woman with allergy to Humulin U. Case report. Przegl Lek. 2005; 62:260–261.
19. Sokup A, Swiatkowski M, Tyloch M, Szymanski W. Outcome of non-pharmacologic treatment in a gestational diabetic woman with high insulin resistance HOMA-IR index and allergy to human insulin. Case report. Ginekol Pol. 2005; 76:403–408.
20. Grammer LC, Roberts M, Buchanan TA, Fitzsimons R, Metzger BE, Patterson R. Specificity of immunoglobulin E and immunoglobulin G against human (recombinant DNA) insulin in human insulin allergy and resistance. J Lab Clin Med. 1987; 109:141–146.
21. Wintermantel C, Fischer N, Muller PH, Velcovsky HG, Lischka G, Eggstein M. Anaphylactic reaction to human insulin. Successful desensitization. Dtsch Med Wochenschr. 1988; 113:1142–1145.
22. Altman JJ, Pehuet M, Slama G, Tchobroutsky C. Three cases of allergic reaction to human insulin. Lancet. 1983; 2:524.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download