Abstract
Background
Selenoprotein P (SEPP1) and fetuin-A, both circulating liver-derived glycoproteins, are novel biomarkers for insulin resistance and nonalcoholic fatty liver disease. However, the effect of exendin-4 (Ex-4), a glucagon-like peptide-1 receptor agonist, on the expression of hepatokines, SEPP1, and fetuin-A, is unknown.
Methods
The human hepatoma cell line HepG2 was treated with palmitic acid (PA; 0.4 mM) and tunicamycin (tuni; 2ug/ml) with or without exendin-4 (100 nM) for 24 hours. The change in expression of PA-induced SEPP1, fetuin-A, and endoplasmic reticulum (ER) stress markers by exendin-4 treatment were evaluated using quantitative real-time reverse transcription polymerase chain reaction and Western blotting. Transfection of cells with AMP-activated protein kinase (AMPK) small interfering RNA (siRNA) was performed to establish the effect of exendin-4-mediated AMPK in the regulation of SEPP1 and fetuin-A expression.
Results
Exendin-4 reduced the expression of SEPP1, fetuin-A, and ER stress markers including PKR-like ER kinase, inositol-requiring kinase 1α, activating transcription factor 6, and C/EBP homologous protein in HepG2 cells. Exendin-4 also reduced the expression of SEPP1 and fetuin-A in cells treated with tunicamycin, an ER stress inducer. In cells treated with the AMPK activator 5-aminoidazole-4-carboxamide ribonucleotide (AICAR), the expression of hepatic SEPP1 and fetuin-A were negatively related by AMPK, which is the target of exendin-4. In addition, exendin-4 treatment did not decrease SEPP1 and fetuin-A expression in cells transfected with AMPK siRNA.
Selenoprotein P (SeP, SEPP1) is a glycoprotein that is mainly expressed in the liver and detected in plasma. SEPP1 plays a role in the transfer of selenium from the liver to plasma and other tissues. SEPP1 transcription is stimulated by interactions among the transcription factors forkhead box protein O1 (FoxO1), hepatocyte nuclear factor 4 (HNF4) α, and peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α, which stimulate hepatic gluconeogenic gene expression. On the other hand, SEPP1 is attenuated by insulin [12]. Serum and hepatic SEPP1 levels reportedly have a strong positive relationship with the development of metabolic diseases [345]. In both animal and clinical studies, SEPP1 has been shown to induce insulin resistance due to dysregulation of insulin signaling and glucose metabolism in the liver and skeletal muscle [3]. Moreover, serum SEPP1 levels are inversely related to blood adiponectin levels in patients with type 2 diabetes, whereas increased adiponectin levels have been observed in SEPP1-knockout mice [6].
Fetuin-A (α2-Heremans-Schmid glycoprotein), a 64-kDA circulating liver-derived glycoprotein, serves as a biomarker for insulin resistance, nonalcoholic fatty liver disease (NAFLD), and cardiovascular disease [789]. Fetuin-A levels are high in human hepatocytes exposed to high levels of palmitic acid (PA), glucose, or endoplasmic reticulum (ER) stress activator thapsigargin [10]. Fetuin-A inhibits insulin-induced IRS-1 tyrosine phosphorylation, thus aggravating insulin resistance [11]. Moreover, fetuin-A-knockout mice exhibit enhanced glucose clearance, improved insulin sensitivity, and resistance to dietary fat-induced weight gain [12].
Exendin-4 (Ex-4), a potent glucagon-like peptide-1 (GLP-1) receptor agonist, is an incretin mimetic capable of relieving insulin resistance. It can decrease plasma glucose and triglyceride levels and increase high density lipoprotein cholesterol levels. The insulin-sensitizing effect of exendin-4 has been reported in human and animal models of insulin resistance [1314]. Exendin-4 also improves metabolic syndrome via modulation of the production and release of various cytokines associated in insulin resistance, oxidative stress, apoptosis, and inflammation [15]. GLP-1 analogue reportedly increases the level of the hepatokine fibroblast growth factor 21, which is involved in insulin sensitivity and glucose and lipid homeostasis [16]. However, the effect of exendin-4 on the expression of the novel hepatokines SEPP1 and fetuin-A remains unknown. Therefore, we evaluated changes in the expression levels of SEPP1 and fetuin-A after administration of exendin-4 under ER stress.
PA, tunicamycin (Tuni), exendin-4, tauroursodeoxycholic acid (TUDCA), and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies to fetuin-A, inositol-requiring enzyme-1α (IRE1α), CCAAT/enhancer binding homologous protein (CHOP), and β-actin were purchased from Cell Signaling Technology (Danvers, MA, USA). SEPP1 and phosphor-IRE1α (P-IRE1α) antibodies were purchased from Abcam (Cambridge, MA, USA). PKR-like endoplasmic reticulum kinase (PERK), phosphor-PERK (P-PERK), activating transcription factor 6 (ATF6), X-box binding protein 1 (XBP-1) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The HepG2 human hepatoma cell line (ATCC, Manassas, VA, USA) was cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco, Grand Island, NY, USA). At approximately 85% confluence in six-well plates, cells were exposed to the ER stress inducers PA and Tuni. After 24 hours, cultured cells were treated with 100 nM exendin-4, 200 µM TUDCA, and 1 mM AICAR, after which they were incubated for 24 hours.
For gene knockout, the cells were transfected with 10 nM small interfering RNA (siRNA) of AMP-activated protein kinase (AMPK) and scrambled control siRNA (Santa Cruz) using the lipofectamine RNAiMAX reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). After incubation for 24 hours, the transfected cells were treated with exendin-4 (100 nM) for 24 hours.
Total RNA was isolated from the cells using Trizol reagent (Invitrogen) to measure the messenger RNA (mRNA) levels of the SEPP1 and fetuin-A genes. Total RNA (2 µg) was reverse-transcribed to complementary DNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). After cDNA synthesis, quantitative real-time PCR was performed using SYBR green (Roche, Lewis, UK) and specific primers (Bioneer Co., Daejeon, Korea) according to the manufacturers' instructions. To normalize the expression of the target genes, the expression of β-actin (Actb) was used as an endogenous control in the comparative Ct method (2-delta delta Ct).
Cells were lysed in ice-cold RIPA buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails (Sigma-Aldrich). The lysates were incubated on ice for 30 minutes, and then centrifuged at 13,000 rpm for 20 minutes at 4℃. The protein concentration of the supernatant was quantified using the Bradford protein assay (Bio-Rad Protein Assay, BioRad, Hercules, CA, USA) with bovine serum albumin standard (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein (20 µg) were electrophoresed on 4% to 12% Bis-Tris Nupage gels (Invitrogen) and transferred to polyvinylidene difluoride membranes using the iBlot Dry Blotting System (Invitrogen). After transfer, the membranes were blocked in 5% bovine serum albumin /Tris buffered saline with Tween-20 buffer for 1 hour, and then incubated overnight at 4℃ with antibodies to SEPP1, fetuin-A, IRE1α, P-IRE1α, PERK, P-PERK, ATF6, XBP-1, CHOP, and β-actin followed by incubation with horseradish peroxidase conjugated secondary antibodies. Immunoreactive bands were visualized with enhanced chemiluminescence Western blotting detection reagents (GE Healthcare, Chalfont St. Giles, UK).
The expression of the SEPP1 and fetuin-A genes was higher in cells treated with PA alone than in the untreated controls, and significantly decreased with exendin-4 treatment in cells that did and did not undergo PA treatment (Fig. 1A). In addition, the expression of proteins exhibited a trend similar to that of their respective protein transcripts (Fig. 1B).
PA, a saturated fatty acid, disrupts ER homeostasis [17], potentially leading to diabetes and hepatic steatosis. To evaluate the effect of exendin-4 on PA-induced ER stress in hepatocyte cells, we examined the effect of exendin-4 on the expression of the ER stress markers IRE1α, PERK, ATF6, and CHOP. Cells exposed to PA displayed higher P-IRE1α, P-PERK, ATF6, and CHOP protein levels, whereas PA-induced increases in the ER stress marker protein levels were reversed in cells treated with exendin-4 (Fig. 2). These results suggest that exendin-4 reduces the expression of PA-induced increases in SEPP1 and fetuin-A, and that exendin-4 improves PA-induced ER stress.
To examine whether increased expression of SEPP1 and fetuin-A secondary to PA treatment is associated with ER stress, HepG2 cells were pretreated with Tuni, an ER stress inducer, followed by the addition of TUDCA, an ER stress inhibitor, or exendin-4. As shown in Fig. 3, the expression of SEPP1 and fetuin-A mRNA as well as XBP-1, a marker for ER stress, was significantly higher in cells treated with Tuni than in the untreated controls. In contrast, supplementation of TUDCA in cells exposed to Tuni completely abolished the effect of Tuni on the expression of these genes. Interestingly, the expression of XBP-1, SEPP1, and fetuin-A in cells treated with exendin-4 exhibited levels similar to those in cells treated with TUDCA. These data suggest that exendin-4 has a protective effect against ER stress, and that exendin-4 attenuates the expression of the SEPP1 and fetuin-A genes by relieving ER stress.
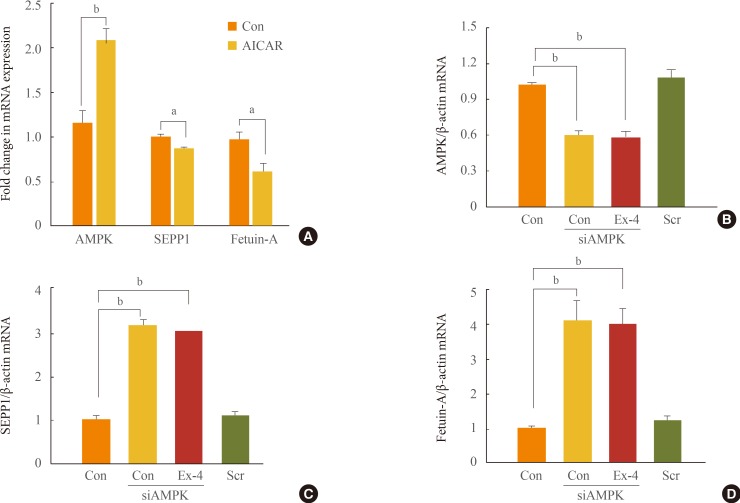
The AMPK activator AICAR can inhibit fatty acid-induced ER stress [18]. SIRT1-AMPK signaling induces a potent pro-tective effect of exendin-4 against fatty liver disease [19]. In this study, we have demonstrated that AICAR downregulates the expression of the SEPP1 and fetuin-A genes (Fig. 4A). We also examined whether the inhibitory effect of exendin-4 on the expression of ER stress-induced SEPP1 and fetuin-A is mediated by AMPK. The expression of AMPK increased in HepG2 cells treated with exendin-4 [19]. However, when the expression of AMPK was inhibited by AMPK siRNA, the expression of SEPP1 and fetuin-A in cells treated with exendin-4 did not decrease (Fig. 4B-D). These results suggest that exen-din-4 inhibits expression of ER stress-induced SEPP1 and fetuin-A via stimulation of AMPK. Activation of AMPK may mediate an inhibitory effect of exendin-4 on ER stress-induced hepatokines, such as SEPP1 and fetuin-A.
We have demonstrated that the expression of SEPP1 and fetuin-A is significantly higher in hepatocytes treated with PA, which induces upregulation of ER stress. On the other hand, exendin-4 treatment decreases the expression of these genes via improvement of ER stress by increasing AMPK.
Recent studies have reported that the hepatokines SEPP1 and fetuin-A can be therapeutic targets of type 2 diabetes mellitus and NAFLD [352021]. Serum SEPP1 and fetuin-A levels are reportedly associated with metabolic syndrome, which is in turn associated with hypoadiponectinemia [62122]. While patients with obesity and NAFLD show significantly increased SEPP1 and fetuin-A levels [423], SEPP1-knockout mice exhibit improved insulin sensitivity in liver and muscle and attenuated adipocyte hypertrophy [3]. Mao and Teng [24] reported that an increased plasma SEPP1 level is the result, rather than the cause of glucose dysregulation, although further studies are needed. In the present study, we demonstrated that the expression of SEPP1 and fetuin-A increased in hepatocytes treated with PA, which can induce fatty liver disease and hepatic insulin resistance [1925], and decreased in hepatocytes treated with exendin-4, which exhibits antidiabetic actions through the GLP-1 receptor. These results are consistent with those of previous studies showing that SEPP1 and fetuin-A are novel biomarkers for metabolic disorders, including obesity, diabetes, and hepatic steatosis [41026].
ER stress plays a crucial role in obesity, insulin resistance [27], and NAFLD [28]. The ER stress response is triggered by different stimuli such as oxidative stress, hypoxia, enhanced protein synthesis, high levels of glucose and saturated fatty acids, and high levels of ER stress inducers, such as thapsigargin and Tuni [2930]. The ER stress response also stimulates ER stress sensors such as ATF6, phosphorylation of PERK and IRE-1α [31], and ER stress-induced transcription factors, such as CHOP and XBP-1 [3233]. In a previous study, we demonstrated that exendin-4 has a protective effect against PA-induced ER stress in hepatocytes [34]. In cells treated with PA, changes in the expression of the ER stress markers, SEPP1 and fetuin-A showed similar patterns, and exendin-4 treatment in cells pretreated with PA or Tuni significantly reduced the expression of these genes. In addition, relieving ER stress by exendin-4 induced decreased expression of the SEPP1 and fetuin-A genes. Thus, these results suggest that decreased expression of SEPP1 and fetuin-A in cells treated with exendin-4 may be associated with improvement of ER stress by exendin-4.
SEPP1 expression is reportedly stimulated by the interaction between the transcription factors FoxO1 and HNF4α, and the coactivator PGC-1α, which stimulates hepatic gluconeogenic gene expression. Jung et al. [35] reported that adiponectin suppresses hepatic SEPP1 through AMPK-mediated phosphorylation of FoxO1a, and ameliorates hepatic fetuin-A through AMPK-mediated reduction of nuclear factor-κB activity [36]. Exendin-4 upregulated the expression of adiponectin and adiponectin receptor 2, and improved hepatic steatosis via SIRT1/AMPK signaling in mice models of diet-induced obesity [19]. The current study suggests that SEPP1 and fetuin-A decreased upon treatment with exendin-4 and the AMPK activator AICAR, whereas when the AMPK gene was silenced with specific siRNA, the SEPP1 and fetuin-A expression did not decrease with exendin-4 treatment. These data suggest that the effect of exendin-4 on the regulation of hepatic SEPP1 and fetuin-A may be mediated by AMPK.
However, the regulatory mechanism of AMPK action on the expression of the SEPP1 and fetuin-A genes remains unclear. Unlike the contention by Jung et al. [35] that salsalate- and adiponectin-mediated AMPK suppresses FoxO1 activity, which is positively associated with SEPP1, Takayama et al. [37] reported that a decrease in the SEPP1 level by treatment with the AMPK activator metformin is dependent on FoxO3a, but not on FoxO1. Moreover, the mechanism of AMPK action on the regulation of fetuin-A expression is poorly defined. Further studies are needed to clarify the effect of exendin-4 on the regulation of hepatic SEPP1 and fetuin-A by treatment with AMPK.
In conclusion, this study demonstrated that PA-induced ER stress stimulates the novel hepatokines SEPP1 and fetuin-A. This study also showed that exendin-4 can suppress the expression of hepatic SEPP1 and fetuin-A via improvement of ER stress by AMPK. Further studies are needed to clarify the details of the mechanism by which exendin-4-induced AMPK controls SEPP1 and fetuin-A.
ACKNOWLEDGMENTS
This study was supported by a National Research Foundation (NRF) grant funded by the Korean government (NRF-2013R1A 1A2063069) and by a grant from Yuhan Corporation (S-2014-1957-000).
References
1. Walter PL, Steinbrenner H, Barthel A, Klotz LO. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008; 365:316–321. PMID: 17986386.

2. Speckmann B, Walter PL, Alili L, Reinehr R, Sies H, Klotz LO, Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1alpha with FoxO1a and hepatocyte nuclear factor 4alpha transcription factors. Hepatology. 2008; 48:1998–2006. PMID: 18972406.
3. Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, Sakurai M, Yamashita T, Mizukoshi E, Yamashita T, Honda M, Miyamoto K, Kubota T, Kubota N, Kadowaki T, Kim HJ, Lee IK, Minokoshi Y, Saito Y, Takahashi K, Yamada Y, Takakura N, Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010; 12:483–495. PMID: 21035759.

4. Choi HY, Hwang SY, Lee CH, Hong HC, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Increased selenoprotein P levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab J. 2013; 37:63–71. PMID: 23439771.

5. Yang SJ, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011; 96:E1325–E1329. PMID: 21677040.

6. Misu H, Ishikura K, Kurita S, Takeshita Y, Ota T, Saito Y, Takahashi K, Kaneko S, Takamura T. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PLoS One. 2012; 7:e34952. PMID: 22496878.

7. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006; 29:853–857. PMID: 16567827.
8. Haukeland JW, Dahl TB, Yndestad A, Gladhaug IP, Loberg EM, Haaland T, Konopski Z, Wium C, Aasheim ET, Johansen OE, Aukrust P, Halvorsen B, Birkeland KI. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol. 2012; 166:503–510. PMID: 22170794.

9. Zhao ZW, Lin CG, Wu LZ, Luo YK, Fan L, Dong XF, Zheng H. Serum fetuin-A levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes. Biomarkers. 2013; 18:160–164. PMID: 23410047.

10. Ou HY, Wu HT, Hung HC, Yang YC, Wu JS, Chang CJ. Endoplasmic reticulum stress induces the expression of fetuin-A to develop insulin resistance. Endocrinology. 2012; 153:2974–2984. PMID: 22619360.

11. Srinivas PR, Wagner AS, Reddy LV, Deutsch DD, Leon MA, Goustin AS, Grunberger G. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993; 7:1445–1455. PMID: 7906861.

12. Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, Jen KL, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002; 51:2450–2458. PMID: 12145157.

13. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006; 43:173–181. PMID: 16374859.
14. Lam S, See S. Exenatide: a novel incretin mimetic agent for treating type 2 diabetes mellitus. Cardiol Rev. 2006; 14:205–211. PMID: 16788334.
15. Dorecka M, Siemianowicz K, Francuz T, Garczorz W, Chyra A, Klych A, Romaniuk W. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacol Rep. 2013; 65:884–890. PMID: 24145082.

16. Yang M, Zhang L, Wang C, Liu H, Boden G, Yang G, Li L. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012; 7:e48392. PMID: 23152772.

17. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006; 291:E275–E281. PMID: 16492686.

18. Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5’ monophosphate-activated protein kinase activation. Endocrinology. 2010; 151:576–585. PMID: 19952270.

19. Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Lee WY. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PLoS One. 2012; 7:e31394. PMID: 22363635.

20. Malin SK, Del Rincon JP, Huang H, Kirwan JP. Exercise-induced lowering of fetuin-A may increase hepatic insulin sensitivity. Med Sci Sports Exerc. 2014; 46:2085–2090. PMID: 24637346.

21. Ishibashi A, Ikeda Y, Ohguro T, Kumon Y, Yamanaka S, Takata H, Inoue M, Suehiro T, Terada Y. Serum fetuin-A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb. 2010; 17:925–933. PMID: 20543523.

22. Hennige AM, Staiger H, Wicke C, Machicao F, Fritsche A, Haring HU, Stefan N. Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One. 2008; 3:e1765. PMID: 18335040.

23. Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008; 93:4479–4485. PMID: 18728159.

24. Mao J, Teng W. The relationship between selenoprotein P and glucose metabolism in experimental studies. Nutrients. 2013; 5:1937–1948. PMID: 23760059.

25. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009; 284:14809–14818. PMID: 19332540.

26. Jung CH, Kim BY, Kim CH, Kang SK, Jung SH, Mok JO. Associations of serum fetuin-A levels with insulin resistance and vascular complications in patients with type 2 diabetes. Diab Vasc Dis Res. 2013; 10:459–467. PMID: 23811603.

27. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004; 306:457–461. PMID: 15486293.

28. Liu J, Jin X, Yu CH, Chen SH, Li WP, Li YM. Endoplasmic reticulum stress involved in the course of lipogenesis in fatty acids-induced hepatic steatosis. J Gastroenterol Hepatol. 2010; 25:613–618. PMID: 19929925.

29. Alhusaini S, McGee K, Schisano B, Harte A, McTernan P, Kumar S, Tripathi G. Lipopolysaccharide, high glucose and saturated fatty acids induce endoplasmic reticulum stress in cultured primary human adipocytes: salicylate alleviates this stress. Biochem Biophys Res Commun. 2010; 397:472–478. PMID: 20515657.

30. Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010; 107:1071–1082. PMID: 21030724.

31. Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005; 74:739–789. PMID: 15952902.
32. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001; 107:881–891. PMID: 11779464.

33. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999; 339(Pt 1):135–141. PMID: 10085237.

34. Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014; 19:649–656. PMID: 24446069.

35. Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn BS, Baik SH, Choi KM. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein P through the AMPK-FOXO1α pathway. PLoS One. 2013; 8:e66529. PMID: 23825542.

36. Jung TW, Youn BS, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Kim BH, Baik SH, Choi KM. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013; 86:960–969. PMID: 23948064.

37. Takayama H, Misu H, Iwama H, Chikamoto K, Saito Y, Murao K, Teraguchi A, Lan F, Kikuchi A, Saito R, Tajima N, Shirasaki T, Matsugo S, Miyamoto K, Kaneko S, Takamura T. Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J Biol Chem. 2014; 289:335–345. PMID: 24257750.

Fig. 1
Exendin-4 (Ex-4) reduced the expression of selenoprotein P (SEPP1) and fetuin-A in HepG2 cells treated with palmitic acid (PA). HepG2 cells were incubated in the presence or absence of PA-containing medium, and treated with or without 100 nM Ex-4 for 24 hours. (A, B) The expression of SEPP1 and fetuin-A was analyzed using quantitative real-time reverse transcription polymerase chain reaction and Western blotting, and the data were normalized based on the β-actin. Con, control; mRNA, messenger RNA. aP<0.05; bP<0.01.
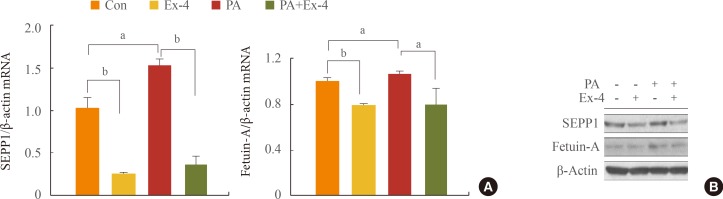
Fig. 2
Exendin-4 (Ex-4) reduced the expression of palmitic acid (PA)-induced endoplasmic reticulum stress markers. HepG2 cells were incubated in the presence or absence of PA-containing medium, and treated with or without 100 nM exendin-4 for 24 hours. Protein expression of inositol-requiring enzyme-1α (IRE1α), PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and CCAAT/enhancer binding homologous protein (CHOP) were analyzed by Western blotting. P-IRE1α, phosphor-IRE1α; P-PERK, phosphor-PERK.
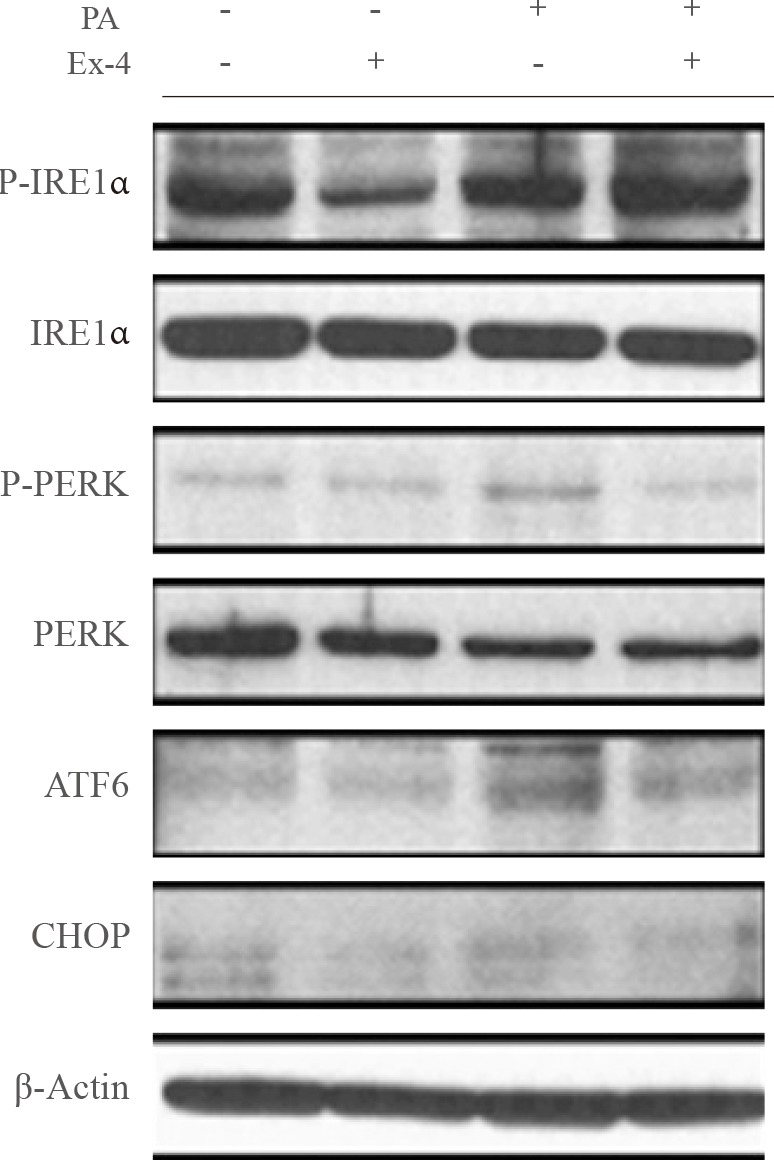
Fig. 3
Expression of selenoprotein P (SEPP1) and fetuin-A increased by endoplasmic reticulum (ER) stress was reversed by exendin-4 (Ex-4). HepG2 cells were treated with tunicamycin (Tuni), an ER stress inducer, for 24 hours, after which tauroursodeoxycholic acid (TUDCA), an ER stress inhibitor, or Ex-4 was added for 24 hours. The gene expression levels of X-box binding protein 1 (XBP-1), SEPP1, and fetuin-A were analyzed using quantitative real-time reverse transcription polymerase chain reaction, and the data were normalized based on the β-actin. Con, control; mRNA, messenger RNA. aP<0.05; bP<0.01.
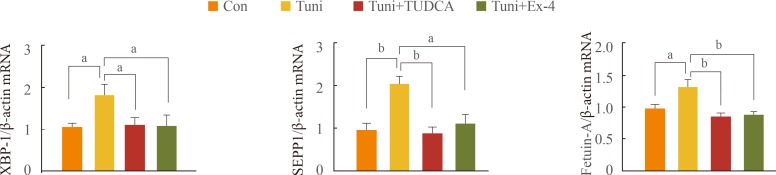
Fig. 4
Expression of selenoprotein P (SEPP1) and fetuin-A in cells treated with exendin-4 (Ex-4) was regulated by AMP-activated protein kinase (AMPK). (A) HepG2 cells were treated with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK activator, for 24 hours. (B-D) Cells were transfected with the specific small interfering RNA (siRNA) for AMPK or scrambled siRNA (Scr) for 24 hours, and then added to a container with or without 100 nM Ex-4 for 24 hours. The expression of AMPK, SEPP1, and fetuin-A messenger RNA (mRNA) was measured using quantitative real-time reverse transcription polymerase chain reaction. Con, control. aP<0.05; bP<0.01.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download