Abstract
Background
Thyroid nodules may harbor cancer in 5% to 15% of cases. Specific clinical and sonographic features predictive of malignancy have been investigated in various populations, but due to differences in epidemiology, risk factors and iodine nutrition status, these predictors may not be valid in the Philippines. This study determined the clinicopathological, biochemical, and sonographic features of thyroid nodules predictive of malignancy among adult Filipino patients at the University of the Philippines-Philippine General Hospital (UP-PGH).
Methods
We reviewed the medical records of Filipino patients ≥19 years of age who underwent thyroid surgery in UP-PGH from 2008 to 2011.
Results
A total of 837 of 1,670 patients (50.1%) were enrolled in the study, which included 417 benign and 420 malignant tumors. The mean age at diagnosis was 38±11 years, with female predominance. Multiple logistic regression analysis showed that the presence of a hard or firm nodule (odds ratio [OR], 58.8, P<0.001; OR, 12.8, P<0.001), presence of microcalcifications (OR, 11.1; P<0.001), irregular margins on ultrasound (OR, 4.5; P<0.001), and absence of associated symptoms (OR, 2.3; P<0.002) increased significantly the likelihood of thyroid malignancy.
Thyroid nodules are the most common endocrine condition worldwide, with prevalence rates ranging from 4% to 23%, depending on the study population and iodine status of the area [1,2,3], and is estimated at 8.9% in the Philippines, based on a national survey conducted in 2008 [4]. Thyroid nodules are a clinically significant health issue because of their probable malignant nature. Reports have shown that thyroid nodules harbor cancer in 5% to 15% of cases [5].
The incidence of thyroid cancer has steadily increased worldwide [6,7] and in the Philippines in the past decades and has become the 15th leading cancer in males and 6th in females among Filipinos [8,9]. In females 15 to 24 years of age, thyroid carcinoma is the most common malignancy [10], and is reported to be even more frequent among migrant Filipino females compared to other Asians and Caucasians living in the same area [11].
Several international guidelines on the diagnosis and management of thyroid nodules and thyroid cancer have identified several high-risk clinical features and ultrasound findings suggestive of thyroid malignancy. These include male gender, age less than 14 or more than 70 years, history of childhood head and neck irradiation, total body irradiation for bone marrow transplantation, family history of thyroid carcinoma or thyroid cancer syndrome in a first-degree relative, exposure to ionizing radiation from fallout, and rapid growth of the nodule and hoarseness [5,12,13,14,15]. Physical findings such as vocal cord paralysis, lateral cervical lymphadenopathy, and fixation of the nodule to the surrounding tissue were also noted to be suggestive of malignancy. Several findings based on the thyroid ultrasound predictive or suggestive of malignancy include microcalcifications, hypoechogenity, increased nodular vascularity, infiltrative margins, shape taller than wide on transverse view, absent halo and suspicious cervical adenopathy.
Data regarding the current status of thyroid cancer in the Philippines are lacking. Studies were conducted in the late 1970s to early 1980s [16,17,18,19,20]; however, only one report has been published since 2000 [8]. The incidence rates and clinical manifestations of thyroid carcinoma were described by the Eufemio and Laudico group in 1979 [21]; incidence rates were 23.3% in males and 13.2% in females. Local symptoms such as pain, dyspnea, dysphagia, and dysphonia are highly associated with thyroid carcinoma, although no statistical analysis was performed. Male gender, age ≥50 years, and hard consistency of nodules in the setting of a nodular nontoxic goiter were also associated with thyroid carcinoma. However, this study was essentially descriptive in nature. Finally, a 5-year retrospective analysis conducted by Quiaoit et al. [22] in 2009 at St. Luke's Medical Center of the association between thyroid function test and thyroid carcinoma revealed that the majority of thyroid carcinomas had normal thyroid stimulating hormone (TSH) levels, with no significant variation among histological types, metastases, and overall staging. However, no data correlating specific sonographic findings with thyroid carcinoma have been reported.
Validating foreign recommendations against local data is an important step in ensuring the best clinical practices for important diseases. Due to the knowledge gap observed locally, analyzing our data to determine applicability in our setting is appropriate. Identifying predictors of malignancy pertinent to Filipinos will facilitate recognition of patients requiring early surgery, while avoiding unnecessary costly surgery in those unlikely to have the disease.
In this study we determined the clinical (history, physical examination, cytopathological findings), biochemical, and sonographic features of thyroid nodules predictive of malignancy among adult Filipino patients examined at the UP-Philippine General Hospital (UP-PGH).
The present retrospective cohort study included Filipino adults ≥19 years of age with thyroid nodules, both known and incidental, who underwent thyroid surgery at the UP-PGH from January 2008 to December 2011. Patients who underwent thyroid surgery for Graves' disease or diffuse toxic goiter without concomitant nodules, thyroid surgery as part of another head-and-neck procedure (i.e., total laryngectomy with prophylactic thyroidectomy) with absence of nodules on final histopathological analysis were excluded. Charts with incomplete data (i.e., incomplete history or absent laboratory results) were also excluded. The final sample size was 834 (417 for each group), with 80% confidence level and 5% relative error based on prevalence of irregular border, the second most commonly associated ultrasonographic finding in thyroid carcinoma [23].
Sample size computation was made through Open Epi Info 6 (Centers for Disease Control and Prevention, Atlanta, GA, USA)
Cases were identified from the admission census of the Department of Otorhinolaryngology and Department of Surgery, and from the histopathology database of the Department of Pathology. A review of the medical records was conducted and data abstraction was performed using a standard data collection form. The study was approved by the Technical Review Board of the Department of Medicine and the University of the Philippines Manila Research Ethics Board.
Demographic data such as age, gender, and pertinent historical findings were recorded, including age at diagnosis, thyroid nodule duration, patient's symptoms (hyperthyroidism, hypothyroidism, compression symptoms), family history of thyroid disease and degree of consanguinity, and history of neck irradiation during childhood. Pertinent physical findings in the neck were obtained from the charts, specifically the number of nodules, size of nodules, consistency (classified as soft, firm, or hard), mobility with deglutition, and presence of cervical lymphadenopathy. The data were based on the findings of at least two physicians (the endocrine fellow in-charge and the surgical resident), who performed a physical examination of the patients upon consultation and admission.
Preoperative TSH results with or without free thyroxine (FT4) or free triiodothyronine (FT3) were recorded. Reference ranges used were as follows: TSH, 0.3 to 3.8 miU/mL; FT4, 11 to 24 pmol/L; FT3, 2.2 to 6.8 pmol/L.
The fine needle aspiration biopsy results were categorized based on the Papanicolaou Society of Cytopathology Task Force on Standard Practice guidelines [24] as inadequate or unsatisfactory, benign, atypical cells present, suspicious for malignancy, or malignant.
Ultrasound findings, such as the composition of the nodules (classified as solid, predominantly solid, mixed solid and cystic, predominantly cystic, cystic), presence or absence of central microcalcifications, echogenicity, presence or absence of halo, presence or absence of peripheral vascularity, cell shape (taller than wide), and presence or absence of suspicious lymph nodes, were recorded.
Histopathological results were classified as benign or malignant; the latter was further classified as differentiated or poorly differentiated thyroid carcinoma.
Statistical analyses were performed using Stata version 10 (Stata Corp., College Station, TX, USA). Frequency and percent distribution were used to describe categorical variables such as gender, presenting manifestation, family history of thyroid disease, childhood history of neck irradiation, ultrasound of thyroid/neck, and postoperative staging. Means and standard deviations were used to describe quantitative/continuous variables such as age, thyroid nodule duration, thyroid function test, and size of the thyroid nodule. Comparison of categorical factors (across outcomes groups; i.e., malignant vs. benign) was performed using chi-square tests while quantitative continuous variables were compared using t tests. Variables found to be significant were evaluated using simple logistic regression and multiple logistic regression analyses to determine specific clinical, biochemical, or sonographic features of thyroid nodules predictive of malignancy in the study population. Statistical significance was set at a P<0.05 with a 95% confidence interval.
A total of 1,670 patients were identified from the combined admission census of the Department of Otorhinolaryngology and Department of Surgery from January 2008 to December 2011, the medical charts of 1,482 of whom were retrieved and reviewed. The reason for nonretrieval was a lost/misplaced chart. A total of 837 patients (50.1%) was included in the analysis. The reasons for exclusion of other charts were incomplete historical data and no available laboratory data attached to the chart.
The mean age at diagnosis was 38 years (range, 19 to 73), with female predominance (78% vs. 16%; female:male ratio, 5:1). The thyroid nodule duration ranged from 1 to 360 months with a mean value of 51 months. All patients presented with a palpable goiter. The majority (75.9%) of patients was asymptomatic; 23.8% had hyperthyroid symptoms such as palpitations, weight loss, heat intolerance, and tremors; 12.1% complained of a rapidly enlarging goiter; 7.2% had symptoms of mechanical compression such as hoarseness, dysphagia, and dyspnea; and 0.8% had symptoms of hypothyroidism. The majority of patients did not have a family history of goiter (85.1%). There was no history of childhood head-and-neck irradiation in the study population. Only 13.5% had comorbid conditions, the most common being hypertension, diabetes mellitus, and dyslipidemia, in the older population. The majority presented with a solitary nodule (66.8% vs. 33.2%). The mean size of the nodule was 4.89 cm, with sizes ranging from a 1-cm solitary nodule to a massive 20-cm multinodular goiter. Eighty-seven percent had a doughy to firm mass, while approximately 13% were hard. Only 2.6% had cervical lymphadenopathy upon diagnosis. A majority of patients (87.6%) were euthyroid, with a mean FT4 of 17.14±6.27 pmol/L and TSH of 1.56±0.96 mIU/L. The remaining patients had subclinical hyperthyroidism (5.7%), subclinical hypothyroidism (2.2%) overt hyperthyroidism (2.0%), and hypothyroxinemia (1.6%).
The general composition of the thyroid nodules was solid (46.4%), predominantly solid (39.0%), mixed solid and cystic (5.9%), predominantly cystic (5.6%), and cystic (3.1%). Twenty-eight percent had microcalcifications on thyroid ultrasound, of which 61.5% were centrally located and 38.5% peripherally located. Fifty-nine percent were isoechoic, 36.6% hypoechoic, and 3.6% hyperechoic; 11% had increased central vascularity; and 3.6% presented with suspicious lymphadenopathy.
Histologically benign thyroid nodules were found in 417 patients, and 420 patients had histologically confirmed thyroid carcinoma. The clinical, biochemical, and sonographic characteristics of the two groups are shown in Tables 1,2,3.
Gender, presenting manifestations such as absence of symptoms (asymptomatic), rapidly enlarging thyroid nodule, hyperthyroid symptoms, nodule size, consistency, mobility, presence of cervical lymphadenopathy, nodule composition, microcalcification, echogenecity, and irregular margins showed significant differences between the two groups. Central vascularity, cell shape (taller than wide), and presence of suspicious cervical adenopathy were not included due to lack of data in the majority of patients.
Simple logistic regression analysis was performed on factors with significant differences (Table 4). Male gender (odds ratio [OR], 2.4), absence of symptoms (asymptomatic; OR, 1.5), rapidly enlarging thyroid nodule (OR, 2.6), firm (OR, 12.3) and hard (OR, 103.7) consistency, fixed nodule (OR, 5.0) presence of cervical lymphadenopathies (OR, 4.4), compositions such as predominantly solid (OR, 3.8) and solid (OR, 6.3) nodules, presence of microcalcifications (OR, 7.2), hypoechogenicity (OR, 2.5), and irregular margins (OR, 6.4) were found to significantly increase the likelihood of thyroid malignancy (all P<0.05). Other factors, such as presence of hyperthyroid symptoms (OR, 0.6), nodule size (OR, 0.87), and isoechogenicity (OR, 0.4) on thyroid ultrasound, were significantly less likely to be associated with thyroid malignancy (all P<0.05).
However, on multiple logistic regression analysis, only the presence of hard nodules (OR, 58.8) or firm nodules (OR, 12.8), presence of microcalcifications (OR, 11.1), irregular margins (OR, 4.5), and absence of symptoms (OR, 2.3) were found to significantly increase the likelihood of thyroid malignancy. Ultrasound findings such as hypoechogenicity (OR, 2.3)-as well as nodule consistencies such as solid (OR, 3.5), predominantly solid (OR, 2.4), mixed solid and cystic (OR, 2.3)-were also associated with a high probability of thyroid malignancy, albeit not significantly so (Table 5).
Gender, rapidly enlarging thyroid nodule, fixed nodules and presence of cervical lymphadenopathies were not included in the final model as they were not significant predictors in the multiple regression analysis (Table 5).
This study showed the clinical, biochemical, and sonographic features of thyroid nodules predictive of malignancy. Similar to local studies, patients were usually in their 30s or 40s when diagnosed, and the majority were female [9,21]. The thyroid nodule duration and family history of thyroid disease did not significantly differ between the two groups. In contrast to international guidelines, history of childhood neck irradiation was not found in our cohort of patients [5,12,13,14,15]. Additionally, in our study, the nodule size was larger in subjects with benign compared to malignant thyroid nodules. The incidence of thyroid cancer was not significantly different in both solitary and multinodular goiters. In this cohort, most of the patients with thyroid nodules were euthyroid in both groups, a finding consistent with the previous local study [25]. The thyroid function test was not predictive of malignancy. However, a slight increase in the number of patients with hypothyroxinemia (FT4, 3 to 10 pmol/L; normal value, 11 to 24 pmol/L) with normal TSH levels was observed and was associated with large goiters, albeit not significantly so; the exact mechanism underlying this association is unclear at present. This is in contrast to the report by Haymart et al. [25], that the risk of malignancy was higher in patients with TSH concentrations >5.0 mIU/L compared with levels <0.06 mIU/L (52% vs. 16%). Solid nodules were also found more frequently in subjects with thyroid carcinoma, albeit not significantly so.
The findings of this study showed that a higher likelihood of thyroid malignancy was associated with the absence of associated symptoms (asymptomatic), presence of firm to hard nodules, and findings of microcalcifications and irregular margins on thyroid ultrasound. These were significantly predictive of thyroid carcinoma, both in the simple logistic and multiple regression analyses, with the hard nodules showing the highest OR (58.8), which is in agreement with the published international guidelines [5].
Based on the results, our report validated the recommendations of several international guidelines for our patients. Identification of such factors can help the clinician distinguish patients with a higher risk of thyroid malignancy, in whom early surgery is recommended to prevent further spread of the disease.
This study had several limitations. First, as this was a retrospective chart review, availability of complete records of the necessary information was not guaranteed; this led to exclusion from the analysis of several charts; approximately half of the reviewed charts were not included due to lack of data. Second, the units used to measure the thyroid hormones differed among the laboratories. Third, reporting of the ultrasound findings was not standardized, which prevented evaluation of other parameters as they were not recorded in the official results. Interobserver variability may also be a limitation since the ultrasound was performed in different laboratories without validation by an independent party. Therefore, we recommend a prospective study on the ultrasound findings of thyroid nodules to evaluate other parameters that could also be associated significantly with thyroid malignancy.
In conclusion, the absence of associated clinical symptoms (asymptomatic), physical findings of firm to hard thyroid nodules, and sonographic findings of microcalcifications and irregular margins were significantly predictive of thyroid malignancy.
Factors such as the presence of rapidly enlarging thyroid nodule, fixation of the nodule on the surrounding tissue, and the composition and hypoechogenicity of the thyroid nodule on ultrasound were also associated with an increased likelihood of malignancy, but did not reach statistical significance in a multivariate analysis.
Figures and Tables
Table 1
Comparison of Patients with Benign and Malignant Thyroid Nodules Based on their Clinical and Demographic Characteristics (n=837)
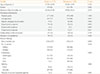
Table 2
Comparison of Patients with Benign and Malignant Thyroid Nodules Based on Their Biochemical and Cytopathological Characteristics

ACKNOWLEDGMENTS
The authors thank the Endocrine Society (ENDO 2013), the Seoul International Congress of Endocrinology and Metabolism (SICEM 2013) for accepting the abstract for poster presentation and the Philippine College of Physicians (PCP) for awarding this as the Best Paper in the Retrospective Category of the Young Investigators Award 2013.
References
1. Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, Braverman LE, Clark OH, McDougall IR, Ain KV, Dorfman SG. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer: American Thyroid Association. Arch Intern Med. 1996; 156:2165–2172.
2. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995; 43:55–68.
3. Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009; 107:72–77.
4. Carlos-Raboca J, Jimeno CA, Kho SA, Andag-Silva AA, Jasul GV Jr, Nicodemus NA Jr, Cunanan EC, Duante CA. The Philippine Thyroid Diseases Study (PhilTiDeS 1): prevalence of thyroid disorders among adults in the Philippines. J Asean Fed Endocr Soc. 2012; 27:27–33.
5. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
6. Pacini F. Changing natural history of differentiated thyroid cancer. Endocrine. 2012; 42:229–230.
7. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009; 20:525–531.
8. Laudico AV, Mirasol-Lumague MR, Mapua CA, Uy GB, Toral JA, Medina VM, Pukkala E. Cancer incidence and survival in Metro Manila and Rizal province, Philippines. Jpn J Clin Oncol. 2010; 40:603–612.
9. De La Pena AS. Thyroid cancer in the Philippines: an update. Acta Med Philipp. 1993; 29:38–42.
10. Department of Health (DOH). Department of Health Statistics [Internet]. Manila: Department of Health (DOH);1998. cited 2012 Apr 16. Available from: http://www.doh.gov.ph.
11. Haselkorn T, Stewart SL, Horn-Ross PL. Why are thyroid cancer rates so high in southeast asian women living in the United States? The bay area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2003; 12:144–150.
12. British Thyroid Association. Royal College of Physicians of London. Guidelines for the management of thyroid cancer. 2nd ed. London: Royal College of Physicians [in association with] British Thyroid Association;2007.
13. Cobin RH, Gharib H, Bergman DA, Clark OH, Cooper DS, Daniels GH, Dickey RA, Duick DS, Garber JR, Hay ID, Kukora JS, Lando HM, Schorr AB, Zeiger MA. Thyroid Carcinoma Task Force. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma: American Association of Clinical Endocrinologists: American College of Endocrinology. Endocr Pract. 2001; 7:202–220.
14. Pacini F, Castagna MG, Brilli L, Pentheroudakis G. ESMO Guidelines Working Group. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21:Suppl 5. v214–v219.
15. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JWA, Wiersinga W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006; 154:787–803.
16. Hermosisima GO. Carcinoma of the thyroid: its prevalence in our area. Philipp J Surg Surg Spec. 1966; 21:270–273.
17. Purisima CO. A clinico-pathological analysis of surgical thyroid cases in the Veterans Memorial Hospital during a ten-year period (1961-1970). [place unknown]: HERDIN;2014. cited 2012 Apr 16. Available from: http://www.herdin.ph/index.php/component/herdin/?view=research&cid=31786.
18. Tipayno MJ. A comparative review on the prevalence of thyroid malignancy in the Cordillera and lowland regions. Philipp J Otolaryngol Head Neck Surg. 2002; 17:160–162.
19. Hermosisima GO. Carcinoma of the thyroid: its prevalence in our area. Philipp J Surg Surg Spec. 1966; 21:270–273.
20. Rivera NV, Lagman CV. The incidence of thyroid carcinoma and complications of thyroid surgery at Ospital ng Maynila: a five-year study [Internet]. [place unknown]: HERDIN;2014. cited 2012 Apr 16. Available from: http://www.herdin.ph/index.php/component/herdin/?view=research&cid=19469.
21. Laudico AV, Eufemio GG, Liquete MJ. Clinical Manifestation of thyroid carcinoma among Filipinos. Philipp J Surg Spec. 1979; 34:159–167.
22. Quiaoit SS, Jasul GV Jr, Quimpo JA. Thyroid function in thyroid carcinoma: a 5-year retrospective analysis. Phil J Int Med. 2009; 47:65–70.
23. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008; 37:401–417.
24. The Papanicolaou. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. Diagn Cytopathol. 1997; 17:239–247.
25. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008; 93:809–814.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download