Abstract
Background
Proinflammatory cytokines are one of the causes of diabetes mellitus. However, the exact molecular mechanism by which proinflammatory cytokines induce β-cell death remains to be clearly elucidated. Glucagon-like peptide-1 (GLP-1) affects the stimulation of insulin secretion and the preservation of β-cells. Additionally, it may exert an antiapoptotic effect on β cells; however, the mechanism underlying this effect has yet to be demonstrated. Therefore, we investigated the protective effects of GLP-1 in endoplasmic reticulum (ER)-mediated β-cell apoptosis using proinflammatory cytokines.
Methods
To induce ER stress, hamster insulin-secreting tumor (HIT)-T15 cells were treated using a mixture of cytokines. Apoptosis was evaluated via MTT assay, Hoechst 33342 staining, and annexin/propidium iodide (PI) flow cytometry. The mRNA and protein expression levels of ER stress-related molecules were determined via PCR and Western blotting, respectively. Nitric oxide was measured with Griess reagent. The levels of inducible nitric oxide synthase (iNOS) mRNA and protein were analyzed via real-time PCR and Western blot, respectively. iNOS protein degradation was evaluated via immunoprecipitation. We pretreated HIT-T15 cells with exendin (Ex)-4 for 1 hour prior to the induction of stress.
Results
We determined that Ex-4 exerted a protective effect through nitric oxide and the modulation of ER stress-related molecules (glucose-regulated protein [GRP]78, GRP94, and CCAAT/enhancer-binding protein homologous protein [CHOP]) and that Ex-4 stimulates iNOS protein degradation via the ubiquitination pathway. Additionally, Ex-4 also induced the recovery of insulin2 mRNA expression in β cells.
Figures and Tables
Fig. 1
After exposure to mixture of cytokines, HIT-T15 cells apoptosis increased by doses of cytokines mixture. A. Cells viability was measured with the MTT assay. HIT-T15 cells were pretreated Ex-4 for 1 hour before mixture of cytokines treatment. B. After treated of mixture of cytokine, effects of the Ex-4 on cell viability were measured by MTT assay. C. Proinflammatory cytokines induced apoptotic nuclei reduced via Ex-4. Fixed cells were stained with hoechst 33342 D. Flow cytometric analysis of apoptosis of HIT-T15 cells exposed to 72 hours. *** < 0.001 vs. control cells; ### < 0.001 vs. CTYs alone.
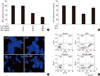
Fig. 2
HIT-T15 cells were pretreated with Ex-4, forskolin, H89. After 1 hour, HIT-T15 cells were treated with mixture of cytokines for 48 hours. A. After treated of mixture of cytokines, effect of the Ex-4, forskolin, H89 on GRP 78, 94 and CHOP were determined by densitometry analysis. B. Western blotting of GRP78,94 and CHOP. * < 0.05, ** < 0.01, *** < 0.001 vs. control cells; # < 0.05, ## < 0.01 vs. CTYs alone; $ < 0.05, $$ < 0.01, $$$ < 0.001 vs. Ex-4 in treated CYTs.

Fig. 3
HIT-T15 cells were pretreated with Ex-4, forskolin, H89. After 1 hour, HIT-T15 cells were treated with mixture of cytokines for 48 hours. A. After treated of mixture of cytokines, effect of the Ex-4, foskolin, H89 on nitric oxide were determined by densitometry analysis. B. Western blotting of iNOS protein. C. After treated of mixture of cytokines, effect of the Ex-4, forskolin, H89 on iNOS mRNA were determined by densitometry analysis. *** < 0.001 vs. control cells; # < 0.05, ### < 0.001 vs. CTYs alone; $$$ < 0.001 vs. Ex-4 in treated CYTs.

Fig. 4
HIT-T15 cells were pretreated with Ex-4. After 1 hour, HIT-T15 cells were treated with mixture of cytokines for 48 hours. A. iNOS protein ubiquitination was increased by Ex-4. B. Expression levels of deubiquitnation enzyme were examined by real-time PCR and western blot. C. real-time PCR of ubiquitin enzyme after treatment cytokines or Ex-4. D. Expression levels of insulin2 mRNA were examined by real-time PCR. ** < 0.01, *** < 0.001 vs. control cells; ## < 0.01, ### < 0.001 vs. CTYs alone; $$ < 0.01, $$$ < 0.001 vs. Ex-4 in treated CYTs.
References
1. Ronn SG, Billestrup N, Mandrup-Poulsen T. Diabetes and suppressors of cytokine signaling proteins. Diabetes. 2007. 56:541–548.
2. Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000. 49:741–748.
3. Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002. 3:411–421.
4. Kim JY, Lee SK, Baik HW, Lee KH, Kim HJ, Park KS, Kim BJ. Protective effects of glucagon like peptide-1 on HIT-T15 beta cell apoptosis via ER stress induced by 2-deoxy-D-glucose. Korean Diabetes J. 2008. 32:477–487.
5. Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002. 51:311–316.
6. Sarkar SA, Kutlu B, Velmurugan K, Kizaka-Kondoh S, Lee CE, Wong R, Valentine A, Davidson HW, Hutton JC, Pugazhenthi S. Cytokine-mediated induction of anti-apoptotic genes that are linked to nuclear factor kappa-B (NF-kappaB) signalling in human islets and in a mouse beta cell line. Diabetologia. 2009. 52:1092–1101.
7. Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004. 145:5087–5096.
8. Pugazhenthi U, Velmurugan K, Tran A, Mahaffey G, Pugazhenthi S. Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: potential therapeutic benefits in diabetic patients. Diabetologia. 2010. 53:2357–2368.
9. Cardozo AK, Kruhoffer M, Leeman R, Orntoft T, Eizirik DL. Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes. 2001. 50:909–920.
10. Parkash J. Inflammatory cytokine signaling in insulin producing beta-cells enhances the colocalization correlation coefficient between L-type voltage-dependent calcium channel and calcium-sensing receptor. Int J Mol Med. 2008. 22:155–163.
11. Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994. 6:399–406.
12. Kang JH, Chang SY, Jang HJ, Kim DB, Ryu GR, Ko SH, Jeong IK, Jo YH, Kim MJ. Exendin-4 inhibits interleukin-1beta-induced iNOS expression at the protein level, but not at the transcriptional and posttranscriptional levels, in RINm5F beta-cells. J Endocrinol. 2009. 202:65–75.
13. Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001. 2:169–178.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download