1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29.
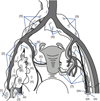
4. Abu-Rustum NR, Barakat RR. Observations on the role of circumflex iliac node resection and the etiology of lower extremity lymphedema following pelvic lymphadenectomy for gynecologic malignancy. Gynecol Oncol. 2007; 106:4–5.
5. Hoffman MS, Parsons M, Gunasekaran S, Cavanagh D. Distal external iliac lymph nodes in early cervical cancer. Obstet Gynecol. 1999; 94:391–394.
6. Ohba Y, Todo Y, Kobayashi N, Kaneuchi M, Watari H, Takeda M, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol. 2011; 16:238–243.
7. Matsumoto K, Yoshikawa H, Yasugi T, Onda T, Nakagawa S, Yamada M, et al. Distinct lymphatic spread of endometrial carcinoma in comparison with cervical and ovarian carcinomas. Cancer Lett. 2002; 180:83–89.
8. Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Risk factors for early and late postoperative complications of patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2006; 124:222–226.
9. Konno Y, Todo Y, Minobe S, Kato H, Okamoto K, Sudo S, et al. A retrospective analysis of postoperative complications with or without para-aortic lymphadenectomy in endometrial cancer. Int J Gynecol Cancer. 2011; 21:385–390.
10. International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology. 2003; 36:84–91.
11. National Cancer Institute Surveillance, Epidemiology, and End Results. SEER stat fact sheets: cervix uteri cancer [Internet]. Bethesda, MD: National Cancer Institute;2015. cited 2016 Apr 13. Available from:
http://seer.cancer.gov/statfacts/html/cervix.html.
12. Todo Y, Yamamoto R, Minobe S, Suzuki Y, Takeshi U, Nakatani M, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol. 2010; 119:60–64.
13. Todo Y, Yamazaki H, Takeshita S, Ohba Y, Sudo S, Minobe S, et al. Close relationship between removal of circumflex iliac nodes to distal external iliac nodes and postoperative lower-extremity lymphedema in uterine corpus malignant tumors. Gynecol Oncol. 2015; 139:160–164.
14. Hareyama H, Hada K, Goto K, Watanabe S, Hakoyama M, Oku K, et al. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: a retrospective study. Int J Gynecol Cancer. 2015; 25:751–757.
15. Hareyama H, Ito K, Hada K, Uchida A, Hayakashi Y, Hirayama E, et al. Reduction/prevention of lower extremity lymphedema after pelvic and para-aortic lymphadenectomy for patients with gynecologic malignancies. Ann Surg Oncol. 2012; 19:268–273.
16. Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999; 85:1547–1554.
17. Yin YJ, Li HQ, Sheng XG, Li XL, Wang X. Distribution pattern of circumflex iliac node distal to the external iliac node metastasis in stage IA to IIA cervical carcinoma. Int J Gynecol Cancer. 2014; 24:935–940.
18. Levenback C, Coleman RL, Burke TW, Lin WM, Erdman W, Deavers M, et al. Lymphatic mapping and sentinel node identification in patients with cervix cancer undergoing radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 2002; 20:688–693.
19. Ogawa S, Kobayashi H, Amada S, Yahata H, Sonoda K, Abe K, et al. Sentinel node detection with (99m)Tc phytate alone is satisfactory for cervical cancer patients undergoing radical hysterectomy and pelvic lymphadenectomy. Int J Clin Oncol. 2010; 15:52–58.
20. Rob L, Strnad P, Robova H, Charvat M, Pluta M, Schlegerova D, et al. Study of lymphatic mapping and sentinel node identification in early stage cervical cancer. Gynecol Oncol. 2005; 98:281–288.
21. Ouldamer L, Marret H, Acker O, Barillot I, Body G. Unusual localizations of sentinel lymph nodes in early stage cervical cancer: a review. Surg Oncol. 2012; 21:e153–7.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download