Abstract
Objective
Concurrent chemoradiation therapy (CCRT) is the standard treatment for locally advanced cervical cancer. Although the optimal chemotherapeutic regimen is not yet defined, previous randomized trials have demonstrated that 5-fluorouracil (5-FU) plus cisplatin every 3 weeks and weekly cisplatin are the most popular regimens. The purpose of this study was to compare the outcomes of weekly CCRT with cisplatin and monthly CCRT with 5-FU plus cisplatin for locally advanced cervical cancer.
Methods
We retrospectively reviewed data from 255 patients with FIGO stage IIB-IVA cervical cancer. Patients were classified into two CCRT groups according to the concurrent chemotherapy: weekly CCRT group, consisted of CCRT with weekly cisplatin for six cycles; and monthly CCRT group, consisted of CCRT with cisplatin and 5-FU every 4 weeks for two cycles followed by additional consolidation chemotherapy for two cycles with the same regimen.
Results
Of 255 patients, 152 (59.6%) patients received weekly CCRT and 103 (40.4%) received monthly CCRT. The mean follow-up period was 39 months (range, 1 to 186 months). Planned CCRT was given to 130 (85.5%) patients in weekly CCRT group and 84 (81.6%) patients in monthly CCRT group, respectively. Severe adverse effects were more common in the monthly CCRT group than in the weekly CCRT group. There were no statistically significant differences in progression-free survival and overall survival between the two groups (p=0.715 and p=0.237).
Cervical cancer is the second most common cancer in women worldwide and the major cause of death particularly in developing countries [1]. Most cervical cancer patients in developed countries are diagnosed at early stage due to the widespread use of effective screening program, and can be successfully treated by primary surgery or radiation therapy when diagnosed at early stage. However, in developing or less developed countries, over 80% of women with cervical cancer are diagnosed at advanced stage, which is significantly associated with poor prognosis [2].
Radiation therapy (RT) alone had been used as a primary treatment for patients with locally advanced-the International Federation of Gynecology and Obstetrics (FIGO) stage IIB to IV-cervical cancer. However it failed both locally and distantly in about 50% of cases, suggesting the need for additional therapeutic modalities [3-5]. Several studies demonstrated that the addition of chemotherapy to RT would enhance the radiation effect [6-9], and five randomized trials in 1990s comparing cisplatin-based concurrent chemoradiation therapy (CCRT) to RT alone showed a significant reduction in the risk of recurrence and death with cisplatin-based CCRT [10-14].
Two CCRT methods-CCRT with weekly cisplatin (weekly CCRT) and cisplatin plus 5-fluorouracil (5-FU) every 3 weeks (monthly CCRT)-were used for locally advanced cervical cancer. In 1999, the Gynecologic Oncology Group (GOG) protocol 120 comparing weekly CCRT and monthly CCRT showed that weekly CCRT was better tolerated and there was no survival difference between the two CCRT modalities [13]. Since then, weekly CCRT has been much widely used as the preferred treatment approach for locally advanced cervical cancer. However, the optimal chemotherapy regimen is not confidently defined, and new combination treatments are under investigation [15].
In our institution, two CCRT modalities have been used as primary treatment for locally advanced cervical cancer since 1994. In the current study, we attempt to compare the therapeutic efficacy, including progression-free survival (PFS) and overall survival (OS), and toxicity of both CCRT modalities-weekly cisplatin and monthly cisplatin plus 5-FU in combination with external pelvic RT.
All patients with FIGO stage IIB to IVA cervical cancer of the uterus treated at Ajou University Hospital from June 1994 to May 2010 were identified and retrospectively reviewed after obtaining approval by the Institutional Review Board.
All patients had invasive cervical cancer histologically confirmed as squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. All patients had their normal hematological, renal, and hepatic functions and performance status within normal limits. None had a history of prior chemotherapy, radiotherapy or surgery. For exact clinical staging, all patients were subjected to a thorough physical examination and pelvic examination, complete blood count (CBC), liver function test, urinalysis, and imaging studies including chest X-ray, pelvic MRI, and/or PET-CT. Cystoscopy and sigmoidoscopy were performed only in patients clinically suspicious of bladder and bowel invasion.
Irradiation consisted of external beam pelvic RT and intracavitary brachytherapy (ICR). External beam pelvic RT dose prescription to the whole pelvis was 4,500 cGy in 25 fractions at the isocenter 4 to 5 weeks apart. A four-field box technique was adopted. After completion of external beam pelvic RT, ICR (30 Gy to point A in five fractions) was performed at 1-week intervals. Patients with suspicious positive para-aortic lymph nodes also received treatment to the para-aortic field with a dose of 4,500 cGy. ICR was performed using tandem and ovoid applicators, selected on the individual's anatomy. The anterior and posterior vagina was packed with radio-opaque gauze to reduce the bladder and rectal exposure. A parametrial boost of 5,400 to 9,000 cGy was given to the involved parametrium at the completion of whole pelvic RT. Based on patient history, physical examination, and CBC, patients were assessed according to the Radiation Therapy Oncology Group (RTOG) criteria for acute toxicities. RT was withheld in the case of an absolute neutrophil count (ANC) less than 1,000/mm3 or platelet count less than 50,000/mm3 and appropriate delays were also allowed in the event of grade 3 to 4 gastrointestinal or genitourinary toxicity.
Patients received 5-FU and cisplatin (FP) chemotherapy as part of monthly CCRT before 2000. FP regimen was administered 4 cycles of FU (1,000 mg/m2/day) on days 2-5 and cisplatin (70 mg/m2/day) on day 1 along with RT for 5 consecutive days at 28-day intervals. The drug was given in an infusion after adequate hydration and antiemetic followed by diuresis. We modified the original monthly CCRT protocol of GOG [10]: after completion of two-cycles of CCRT, the additional one or two cycles of chemotherapy with same FP regimen was given to patients as consolidation chemotherapy. Since 2000, weekly cisplatin in conjunction with RT has been used as the preferred primary treatment. Weekly cisplatin regimen started with a dose of 40 mg/m2 to a maximum of 70 mg on day 1 of external RT, 1 to 4 hours before RT initiation. The Eastern Cooperative Oncology Group (ECOG) toxicity criteria were used for monitoring and documentation of hematological toxicities. Chemotherapy was delayed for an ANC less than 500/mm3, or platelet count less than 50,000/mm3. We injected granulocyte colony stimulating factor (G-CSF) and intravenous antimicrobials only when the patient had a febrile neutropenia, defined as an oral temperature higher than 38.5℃ and persistent neutropenia with serious complications such as pneumonia or any type of progressive infection. We did not administer prophylactic human G-CSF to the patients with an ANC less than 500/mm3.
The response to treatment was evaluated using physical examination, Pap smear, MRI, and/or PET-CT. First follow-up was done at a month after completion of treatment, then every 2-3 months in the first year, every 3-6 months in the second year, and every half a year from the third to the fifth year.
Statistical analysis was performed using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). Clinical and pathologic factors were compared between two groups with Pearson's χ2 test for categorical data, and the Student t-test and for continuous data according to normality. The Kaplan-Meier method was used to construct curves for PFS and OS; and comparison of survival rates was performed using the long-rank test. A significant level of 0.05 was used for all tests.
A total of 260 consecutive patients with locally advanced cervical cancer were identified, and 5 patients who received only RT as a local hemostatic control in order to control profuse vaginal bleeding were excluded. Finally, a total of 255 patients were included in this study.
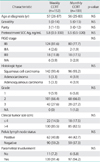
Clinicopathologic characteristics of patients with weekly and monthly CCRT are compared in Table 1. Of 255 patients, 103 (40.4%) patients received monthly CCRT and 152 (59.6%) received weekly CCRT. The age of patients ranged from 26 to 87 years with mean age at diagnosis being 57 years. Majority of patients belonged to stage IIB (81.6% in the weekly CCRT group vs. 77.7% in the monthly CCRT group). Number of patients with squamous cell carcinoma histology was 142 in the weekly CCRT group and 96 in the monthly CCRT group, respectively. The follow-up time of the 255 cases was 1 to 186 months, with a median follow-up time of 39 months. All patients completed their scheduled treatment without death due to toxicities. There were no statistically significant differences of clinicopathological characteristics between the two groups.
The 5-year PFS and OS of patients in the weekly CCRT group versus the monthly CCRT are 74.6% vs. 64.3% and 78% vs. 73%, respectively (Fig. 1). There were no statistically significant differences in PFS and OS between the two groups (p=0.7105 and p=0.237).
Table 2 shows the rate of completion of RT and chemotherapy. Although toxicity-related delay was noted in the two groups, most gastrointestinal and genitourinary toxicities were controlled with supportive care. Most complications appeared after 80% completion of the planned chemotherapy (after 3 cycles of FP or 5 cycles of cisplatin). Chemotherapy was discontinued on patient's request or under the recurrent febrile neutropenia.
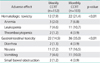
The types and frequencies of adverse effects are shown in Table 3. There were no treatment-related deaths. The major acute toxicities were hematological and gastrointestinal toxicities. Twelve (7.9%) and 22 (21.4%) patients had grade 3-4 hematologic toxicity in the weekly CCRT and monthly CCRT group, respectively (p<0.01). There were no instances of serious late complications requiring laparotomy. No remarkable alopecia was observed, nor did cisplatin-induced neurotoxicity or ototoxicity occur. The grade 3-4 acute gastrointestinal toxicities were more often observed in the monthly CCRT than in the weekly CCRT group, respectively (14.5% vs. 35.0%; p<0.01). Irreversible adverse effects, including persistent electrolyte imbalance and bone marrow failure, were not noted in the both groups.
Patterns of failure are shown in Table 4. During a median follow-up period of 39 months (range, 1 to 186 months), 37 (24.3%) and 23 (22.3%) patients had recurrence, with similar findings observed in both groups.
Since the National Cancer Institute (NCI) consensus stated "...strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiation therapy in women who require radiation therapy for treatment of cervical cancer..." in 1999, CCRT has been established as the standard of care for locally advanced cervical cancer [15,16]. Green et al. [17] suggested in a meta-analysis of 4,580 patients from 19 randomized prospective trials that CCRT was superior to RT alone in controlling both local failure and distant relapse, another meta-analysis reported similar results supporting the NCI statement [18]. Although, from the late 1990s GOG studies, two regimens of 6-cycle weekly cisplatin and 2-cycle FP at 3- to 4-week intervals in concurrent with RT were found to have same efficacy for locally advanced cervical cancer, weekly cisplatin has been recommended as the standard treatment for this disease because of its good tolerability to patients [10,13].
There was a great change of treatment pattern for locally advanced cervical cancer in our institution. Before 2000, the regimen of monthly FP in conjunction with external pelvic RT was a standard CCRT approach. However, our approach was quite different to the regimen proposed by GOG 85 [10]. We modified the CCRT protocol using FP regimen of the GOG with introducing the concept of so-called 'consolidation' chemotherapy. Patients received 70 mg/m2/day of cisplatin on days 1 and 29, followed by 1,000 mg/m2/day of fluorouracil given as a 96-hour infusion on days 1 and 29 during RT, and had the two additional FP chemotherapy without RT after completion of CCRT. After all, a total of 4-cycle FP chemotherapy was given to the patients during and after the planned RT. We hypothesized that the additional chemotherapy might help eradicating micrometastases outside the irradiation fields, sensitizing tumor cells to radiation, and improving overall survival in patients with locally advanced cervical cancer. Several studies have reported on the use of consolidation chemotherapy after CCRT [19-22]. Wong et al. [19] showed that chemoradiation followed by adjuvant chemotherapy with epirubicin had a less frequent distant failure without difference in local failure rate. Vrdoljak et al. [20,21] reported promising results after consolidation chemotherapy with ifosfamide and cisplatin after concomitant chemobrachyradiotherapy. Recently, Choi et al. [22] reported that three more cycles of consolidation FP chemotherapy after CCRT might have a role as a radiosensitizer during the period of delayed radiation effect and lead to an encouraging survival rate, with acceptable toxicity. We used FP regimen as consolidation chemotherapy based on the findings that the addition of 5-FU and cisplatin-based chemotherapy to RT could enhance the radiation effects [6-9]. However, since GOG 120 demonstrated the efficacy of weekly cisplatin-based CCRT compared to monthly CCRT [13] and it was reported that there was no significant effect of 5-FU in either form of combination with cisplatin or alone [23], we changed our treatment strategy and weekly cisplatin CCRT has been our standard primary treatment for locally advanced cervical cancer.
In the present study, the survival outcomes and side effects between weekly and monthly CCRT were compared. To the best of our knowledge, there have been few studies on comparing weekly cisplatin-based CCRT with monthly CCRT with additional consolidation chemotherapy. The PFS and OS rates were similar to or slightly better than those of other previous studies [10,11,13,15]. However there were no statistically significant differences of survival rates and the pattern of failure between the two CCRT approaches. These findings suggest that consolidation FP regimen used in this study might be insufficient to eradicate micrometastases.
The dose, regimen and schedule of chemotherapeutic agents could explain the differences in toxicity, compliance and treatment delays. Kim et al. [24] reported that 73% of patient could receive 6 cycles of cisplatin at a dose of 30 mg/m2, in contrast to 49% in GOG 120 in which 40 mg/m2 of weekly cisplatin was delivered [13]. Similarly, other investigators reported that less than half of women completed the full planned weekly cisplatin at a dose of 40 mg/m2 combined with pelvic RT [25]. Therefore, more patients in the dose reduction group completed full dose of planned chemoradiation with similar survival. Dose reduction may be necessary for reducing acute and late toxicities. However, in multivariate analysis done by Nugent et al. [26] on 118 patients with locally advanced cervical cancer, the number of cisplatin chemotherapy cycles was independently predictive of PFS and OS. Patients who received less than 6 cycles of cisplatin had a worse PFS and OS. Additionally, advanced stage, longer time to RT completion, and absence of brachytherapy were associated with decreased PFS and OS (p<0.05). They recommended all patients with locally advanced cervical carcinoma (LACC) be offered six cycles of cisplatin for optimal dose treatment. In addition to the dose reduction, a randomized trial comparing cisplatin 40 mg/m2 weekly ×6 with cisplatin 75 mg/m2 every 3 weeks ×4 demonstrated that weekly cisplatin regimen had more complete treatment rate and less delayed courses than with three-weekly cisplatin [27]. Therefore, associations between both completion of scheduled dose for improving survivals and dose reduction for minimizing toxicities should be investigated as thoroughly. With regard to completion rates of the planned treatment and treatment-related toxicities in the current study, the cumulative dose of cisplatin in weekly CCRT group was 240 mg/m2 compared to 280 mg/m2 in monthly CCRT group. Grade 3-4 hematologic (21.4% vs. 7.9%) and gastrointestinal toxicities (35.0% vs. 14.0%) were more frequent in the monthly CCRT group. Although there were delays in giving chemotherapy, most patients completed more than 80% of planned cycles of chemotherapy in addition to their scheduled RT, which showed an improved PFS and OS, and our data are comparable to those from others [12,13,24,25,28,29].
There are several limitations-selection, information, and confounding bias-which are inherent in the retrospective nature of this study, and these limit clear conclusions. Although there was no significant difference of various clinical and pathological parameters between weekly and monthly CCRT groups, selection bias may influence our results. Moreover, we admit that a wide-range of follow-up time and censored data could decrease the power of results of the present study. Monthly CCRT was performed as the preferred treatment from 1994 to 1999 and weekly CCRT from 2000 to 2010. The direct comparison of different approaches without adjusting time period also should be considered in interpreting our results. Despite these limitations, the present study may give a matter that merits our sober reflection on consolidation chemotherapy in addition to CCRT through homogenous study populations with consistent treatment and long-term follow-up.
In conclusion, weekly cisplatin with concurrent RT and monthly FP with concurrent RT followed by consolidation FP chemotherapy had similar efficacy for patients with locally advanced cervical cancer, and weekly CCRT is better tolerated. However, since local disease control remains an issue in patients with locally advanced cervical cancer, there is a potential for improvement with newer combinations. Further multicenter randomized trials with a number of newer chemotherapeutic agents are necessary to evaluate the overall oncologic outcomes and quality-of-life.
Figures and Tables
Fig. 1
Progression-free (A) and overall survival (B) according to the type of concurrent chemoradiation therapy (CCRT) in 255 locally advanced cervical cancer patients. The 5-yr progression-free survival and overall survival of patients in the group given weekly CCRT versus monthly CCRT are 74.6% vs. 64.3% and 78% vs. 73%, respectively. FP, 5-fluorouracil plus cisplatin.

Notes
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
2. Denny L. Cervical cancer treatment in Africa. Curr Opin Oncol. 2011. 23:469–474.
3. Jampolis S, Andras EJ, Fletcher GH. Analysis of sites and causes of failures of irradiation in invasive squamous cell carcinoma of the intact uterine cervix. Radiology. 1975. 115:681–685.
4. Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys. 1991. 20:667–676.
5. Perez CA. Radiation therapy in the management of cancer of the cervix. Oncology (Williston Park). 1993. 7:61–69.
6. Byfield JE, Calabro-Jones P, Klisak I, Kulhanian F. Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-Fluorouracil or ftorafur and X rays. Int J Radiat Oncol Biol Phys. 1982. 8:1923–1933.
7. Thomas G, Dembo A, Beale F, Bean H, Bush R, Herman J, et al. Concurrent radiation, mitomycin C and 5-fluorouracil in poor prognosis carcinoma of cervix: preliminary results of a phase I-II study. Int J Radiat Oncol Biol Phys. 1984. 10:1785–1790.
8. Thomas G, Dembo A, Fyles A, Gadalla T, Beale F, Bean H, et al. Concurrent chemoradiation in advanced cervical cancer. Gynecol Oncol. 1990. 38:446–451.
9. Grigsby PW, Graham MV, Perez CA, Galakatos AE, Camel HM, Kao MS. Prospective phase I/II studies of definitive irradiation and chemotherapy for advanced gynecologic malignancies. Am J Clin Oncol. 1996. 19:1–6.
10. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999. 17:1339–1348.
11. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999. 340:1137–1143.
12. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999. 340:1154–1161.
13. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999. 340:1144–1153.
14. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000. 18:1606–1613.
15. Lanciano R, Calkins A, Bundy BN, Parham G, Lucci JA 3rd, Moore DH, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol. 2005. 23:8289–8295.
16. Al-Mansour Z, Verschraegen C. Locally advanced cervical cancer: what is the standard of care? Curr Opin Oncol. 2010. 22:503–512.
17. Green J, Kirwan J, Tierney J, Symonds P, Fresco L, Williams C, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2001. (4):CD002225.
18. Lukka H, Hirte H, Fyles A, Thomas G, Elit L, Johnston M, et al. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer: a meta-analysis. Clin Oncol (R Coll Radiol). 2002. 14:203–212.
19. Wong LC, Ngan HY, Cheung AN, Cheng DK, Ng TY, Choy DT. Chemoradiation and adjuvant chemotherapy in cervical cancer. J Clin Oncol. 1999. 17:2055–2060.
20. Vrdoljak E, Prskalo T, Omrcen T, Situm K, Boraska T, Frleta Ilic N, et al. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: results of a phase II study. Int J Radiat Oncol Biol Phys. 2005. 61:824–829.
21. Vrdoljak E, Omrcen T, Novakovic ZS, Jelavic TB, Prskalo T, Hrepic D, et al. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy for women with locally advanced carcinoma of the uterine cervix: final results of a prospective phase II-study. Gynecol Oncol. 2006. 103:494–499.
22. Choi CH, Lee JW, Kim TJ, Kim WY, Nam HR, Kim BG, et al. Phase II study of consolidation chemotherapy after concurrent chemoradiation in cervical cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2007. 68:817–822.
23. Thomas G, Dembo A, Ackerman I, Franssen E, Balogh J, Fyles A, et al. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol. 1998. 69:137–145.
24. Kim YS, Shin SS, Nam JH, Kim YT, Kim YM, Kim JH, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008. 108:195–200.
25. Serkies K, Jassem J. Concurrent weekly cisplatin and radiotherapy in routine management of cervical cancer: a report on patient compliance and acute toxicity. Int J Radiat Oncol Biol Phys. 2004. 60:814–821.
26. Nugent EK, Case AS, Hoff JT, Zighelboim I, DeWitt LL, Trinkhaus K, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010. 116:438–441.
27. Chumworathayi B, Suprasert P, Charoenkwan K, Srisomboon J, Phongnarisorn C, Siriaree S, et al. Weekly versus three-weekly cisplatin as an adjunct to radiation therapy in high-risk stage I-IIA cervical cancer after surgery: a randomized comparison of treatment compliance. J Med Assoc Thai. 2005. 88:1483–1492.
28. Chen SW, Liang JA, Hung YC, Yeh LS, Chang WC, Lin WC, et al. Concurrent weekly cisplatin plus external beam radiotherapy and high-dose rate brachytherapy for advanced cervical cancer: a control cohort comparison with radiation alone on treatment outcome and complications. Int J Radiat Oncol Biol Phys. 2006. 66:1370–1377.
29. Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002. 20:966–972.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download