Abstract
Objective
The purpose of the present study was to elucidate the incidence of deep venous thrombosis (DVT) before treatment in ovarian cancer and the appropriate cut-off value of D-dimer (DD) for the diagnosis of DVT.
Methods
Between July 2007 and October 2008, eighty seven patients with presumed ovarian cancer (final diagnosis: ovarian cancer, n=59; borderline malignancy, n=28) were enrolled. Measurement of DD levels and subsequent venous ultrasonography were performed before treatment.
Results
The mean DD level was 4.1 µg/mL. Subsequent venous ultrasonography revealed DVT in 14 of 87 (16.1%) patients (ovarian cancer, 12 cases; borderline malignancy, 2 cases). None were found to have developed DVT if they had a DD level of <1.5 µg/mL. If 1.5 µg/mL was used as a cut-off value for DD levels to diagnose DVT, sensitivity, specificity, positive predictive value, and negative predictive value were 100%, 61.6%, 33.3%, and 100%. There was noclinical onset of postoperative pulmonary thromboembolism.
Patients with ovarian cancer have a high risk of developing deep venous thrombosis (DVT) [1]. A symptomatic venous thromboembolism has been reported to correlate with prognosis in ovarian cancer [2]. Therefore, accurate diagnosis of DVT is required to appropriately treat patients with this disease. D-dimer (DD) is known to be a useful molecular marker of blood coagulation and fibrinolysis [3-5]. DD is a specific degradation product resulting from the digestion of cross-linked fibrin by plasmin. High DD levels are to be expected with increased fibrin formation and an efficient fibrinolytic system. The presumption is that DD levels will be elevated in cases of ovarian cancer; however, the levels of DD indicative of DVT remain unclear. Ultrasonography using vein compression and color Doppler reveals deep venous thrombi. The present study sought to evaluate the usefulness of DD levels and subsequent venous ultrasonography in diagnosing DVT prior to the treatment of ovarian cancer, and also to elucidate the incidence of DVT and the appropriate cut-off value for DD to aid in the diagnosis of DVT.
The subjects were 87 patients with presumed ovarian cancer who were seen at the Gynecology Department of the Shizuokas Cancer Center Hospital between July 2007 and October 2008. The data were obtained retrospectively from each patient's medical record. Tumors of 59 patients were histologically diagnosed as ovarian cancer after surgery, and the remaining 28 patients were ovarian borderline malignancy. Peripheral blood samples were collected from all patients and the DD levels were measured on the first visits to our outpatient clinic 2-4 weeks before initial treatment of ovarian cancer. At the same time, physical examination (calf tenderness, distension of collateral veins, edema, swelling of the calf or the whole affected limb) were performed. The plasma DD level was measured using LPIA-ACE DD reagent (Mitsubishi Chemical Medicine Co., Tokyo, Japan) and JIF23 monoclonal antibody. The JIF23 monoclonal antibody, which recognizes the plasmin-digested N-terminus of the γ chain in the D region of fibrin, was used for latex agglutination.
Venous ultrasonography was performed to detect DVT in all patients using a LOGIQ 9 ultrasound scanner (GE Healthcare, Willowick, OH, USA) equipped with a 4-7 MHz transducer. Venous lumina were observed while searching for thrombi by manual compression with a transducer and color Doppler imaging. Patients with DVT detected by venous ultrasonography underwent enhanced computed tomography of the lungs to diagnose pulmonary thromboembolism (PTE).
We performed anticoagulant therapy using unfractionated heparin before initial treatment and after surgery for all patients with DVT. An inferior vena cava filter (IVC-F) was used immediately before surgery to prevent lethal PTE in patients with a deep venous thrombus in proximal veins (1 patient), such as the iliac and femoral veins, or a floating deep venous thrombus in peripheral veins (4 patients).
For patients without DVT, intermittent pneumatic compression was used during and after surgery. Low-molecular-weight heparin was administered after surgery to prevent postoperative venous thromboembolism (VTE).
The protocol for this research project was approved by the Ethics Committee of Shizuoka Cancer Center Hospital and conforms to the Declaration of Helsinki. All patients provided written informed consent. Data are expressed as mean values. The differences between the groups were tested for statistical significance using the Mann-Whitney U-test and the χ2 test. A p-value of less than 0.05 was considered significant. All statistical analyses were performed using an SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
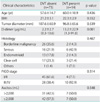
The mean age of all patients was 58.1 years (range, 21 to 85 years) and the mean BMI was 21.9 (range, 16.6 to 33.7). The mean tumor diameter was 105.7 mm (20 to 270 mm). The mean DD level was 4.1 µg/mL (range, 0.2 to 91.8 µg/mL). Subsequent venous ultrasonography revealed DVT in 14 of 87 patients (16.1%). Patients with DVT included 2 patients with ovarian borderline malignancy.
The 87 patients were divided into 2 groups depending on whether they also had (14 patients) or did not have (73 patients) DVT; the results of a comparison of the 2 groups are shown in Table 1. There was no significant difference between the groups in terms of age, BMI, and tumor size. In contrast to a mean DD level of 2.2 µg/mL in the patients who did not have DVT, patients who also had DVT had a significantly higher mean DD level of 13.2 µg/mL. However, differences between the 2 groups in terms of histology, stage of progression, and perioperative volume of ascites were not noted. Moreover, mean levels of DD of FIGO stage I/II were higher than FIGO stage III/IV, but this difference was not statistically significant (Table 2).
In 87 patients, the site where DVT was located was in the left leg in 3 patients, in the right leg in 6, and in both legs in 5. Thirteen patients had DVT in the lower leg, primarily in the veins of the soleus muscle, and only 1 patient had DVT in the femoral vein. PTE was not noted preoperatively in any of the patients. Thirteen patients with DVT were asymptomatic when DVT was found. However, only one patient with DVT in the femoral vein experienced calf tenderness and swelling of the calf.
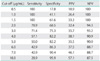
The relationship between DD levels and the incidence of DVT is shown in Table 3. Among the patients, none were found to have developed DVT if they had a DD level of <1.0 µg/mL; however, DVT was noted in 16.1% of patients with 1.0 µg/mL ≤ DD ≤ 3.0 µg/mL, in 21.1% of those with 3.0 µg/mL<DD≤10.0 µg/mL, and in 71.4% of those with a DD level of >10.0 µg/mL. DVT was found in all of the ovarian cancer patients with a DD level of >10.0 µg/mL.
Shown in Table 4 are the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of DD levels in predicting DVT in patients with presumed ovarian cancer. Raising the cut-off value for DD resulted in a higher specificity and PPV and a lower sensitivity and NPV. None were found to have developed DVT if they had a DD level of <1.5 µg/mL. If 1.5 µg/mL was used as a cut-off value for DD levels to diagnose DVT, sensitivity, specificity, PPV, and NPV were 100%, 61.6%, 33.3%, and 100% (Table 3).
A temporary IVC-F was inserted preoperatively in 5 patients. The indications for filter placement were as follows: 1) a femoral deep venous thrombus (1 patient) and 2) a free-floating thrombus (4 patients). Of these 5 patients, one developed PTE postoperatively. This patient had a DD level of 15.8 µg/mL preoperatively; venous ultrasonography of the lower extremities revealed a DVT in the right femoral vein. For this reason, the IVC-F was placed preoperatively and the patient subsequently underwent anticoagulant therapy with heparin. On postoperative day 8, the IVC-F was removed; however, on day 9 the patient developed a sub-massive PTE. After the onset, urokinase was administered in addition to heparin, whereupon the patient's symptoms abated, and the patient was discharged on postoperative day 24. Apart from this patient, no other patient developed postoperative PTE.
Since the publication of the Prevention of Venous Thromboembolism Guidelines of the American College of Chest Physicians (ACCP) [6], postoperative prevention of PTE has become widely prevalent. However, more than 80% of PTE occurs secondary to a DVT in veins in the leg or pelvis, and in more than 40% of the cases of DVT, PTE will develop if the DVT is left untreated [7,8]. Important questions for the future are how to screen preoperatively for DVT and, if DVT is found, what type of PTE prophylaxis should be performed.
In patients with ovarian cancer, a massive tumor forms in the pelvis. As a consequence, venous blood flow in the lower extremities often stagnates, and a large volume of ascites facilitates intravascular hypovolemia and the ready formation of thrombi [9,10]. Moreover, ovarian cancer involves a considerably larger number of cancer cells than other types of cancer; consequently, an extremely large amount of tissue factor (TF), which plays a role in thrombogenesis [11,12], is released by the cancer cells [1]. Given this fact, a large number of patients with ovarian cancer are presumed to have already developed VTE prior to cancer treatment [1]. TF, a lipophilic phospholipoprotein transmembrane receptor with potent procoagulant activity, is expressed in ovarian cancer. This expression is significantly correlated with VTE development in clinical situations. Procoagulant activity with DD elevation may be mediated by TF expression in ovarian cancer [13].
In the present study, we measured DD and performed venous ultrasonography of the lower extremities in all patients with presumed ovarian cancer. DVT was noted in 12 patients with ovarian cancer (20.3%) and 2 patients with borderline malignancy (7.1%). In other words, our results revealed that 1 in 5 patients with ovarian cancer developed DVT preoperatively. No symptoms of DVT were noted in 13 out of 14 patients with DVT. Therefore, physical examination in patients suspected of DVT is of limited value to identify patients with a low or high probability of DVT.
Typically, postoperative intermittent pneumatic compression, as is used to prevent the onset of PTE, is contraindicated when DVT is present. A failure to have screened for DVT in the 14 patients with ovarian cancer in whom DVT was noted preoperatively could have led to inappropriate treatment for the prevention of PTE. In addition, recent research has indicated that among the types of ovarian cancer, DVT often develops in cases of clear cell adenocarcinoma [13-15]. In the present study, however, we found no such high incidence of DVT in cases of clear cell adenocarcinoma.
In diagnostic imaging used to detect DVT, contrast venography is the gold standard; however, its invasiveness, i.e., its use of a contrast material, presents a problem. Venous ultrasonography of the lower extremities is a simple and noninvasive examination and can be performed repeatedly at the patient's bedside. With the B-mode compression technique, the presence or absence of a thrombus shadow and the presence or absence of venous collapse due to the compression of veins in the lower extremities are crucial. An examination is performed while repeatedly compressing the veins and then releasing that pressure; if the examination still fails to clearly indicate whether DVT is present, color Doppler is used [16-18]. Distal muscles are compressed while viewing color Doppler images to ascertain the presence or absence of blood flow around the thrombus. Although ultrasonography of veins in the lower extremities is a simple procedure that can be quickly performed, it does present several problems. For example, diagnosing a deep venous thrombus in veins in the pelvis is difficult, and the ultrasonographer must be experienced in order to diagnose a deep venous thrombus in the femoral vein. Typically, venous ultrasonography of the lower extremities to diagnose DVT has a sensitivity of 97% and a specificity of 94% [19].
The DD levels used to predict DVT generally have a high sensitivity and NPV of 80-100%, but a low specificity and PPV of approximately 20-60% [20,21]. In other words, if DD is at its normal level, then DVT is not present. However, the DD level has a low specificity and PPV; therefore, DVT may not necessarily be present despite a high DD level. In addition, a report based on patients in whom DVT was ruled out because DD was also negative indicated that such patients had a 0.4% probability of developing DVT7. If the cut-off value for patients with cancer is set to 0.5 µg/mL, then their positivity for DD will typically be higher than that of patients without cancer [22]. Thus, caution is required when interpreting DD levels; definitive diagnosis of DVT requires not only measurement of a certain DD level but also some form of diagnostic imaging. From the perspective of cost-effectiveness, an imaging study should be performed when DD is above a certain level. In the present study, the cut-off value for DD in predicting DVT was determined to be 1.5 µg/mL. A suitable cut-off value for detecting DVT in patients with presumed ovarian cancer appears to be 1.5 µg/mL, as this has a 100% sensitivity, a 100% NPV. However, this cut-off value had only a 61.6% specificity and a 33.3% positive predictive value. Therefore, performing venous ultrasonography at DD levels in excess of 1.5 µg/mL might improve the detection rate of DVT.
In conclusion, this study demonstrates that DVT occurs before treatment of ovarian cancer in at least 16.1% of patients. Venous ultrasonography should be performed prior to treatment of ovarian cancer in patients with elevated levels of DD (>1.5 µg/mL). The usefulness of preoperative assessment of DVT needs further confirmation in randomized controlled trials.
Figures and Tables
References
1. Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007. 97:1053–1057.
2. Lim MC, Lee HS, Kang S, Seo SS, Lee BY, Park SY. Minimizing tumor burden by extensive cytoreductive surgery decreases postoperative venous thromboembolism in ovarian clear cell carcinoma. Arch Gynecol Obstet. 2010. 281:329–334.
3. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003. 349:1227–1235.
4. Wells PS, Brill-Edwards P, Stevens P, Panju A, Patel A, Douketis J, et al. A novel and rapid whole-blood assay for D-dimer in patients with clinically suspected deep vein thrombosis. Circulation. 1995. 91:2184–2187.
5. Freyburger G, Trillaud H, Labrouche S, Gauthier P, Javorschi S, Bernard P, et al. D-dimer strategy in thrombosis exclusion--a gold standard study in 100 patients suspected of deep venous thrombosis or pulmonary embolism: 8 DD methods compared. Thromb Haemost. 1998. 79:32–37.
6. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008. 133:6 Suppl. 381S–453S.
7. Heim SW, Schectman JM, Siadaty MS, Philbrick JT. D-dimer testing for deep venous thrombosis: a metaanalysis. Clin Chem. 2004. 50:1136–1147.
8. Huisman MV, Buller HR, ten Cate JW, van Royen EA, Vreeken J, Kersten MJ, et al. Unexpected high prevalence of silent pulmonary embolism in patients with deep venous thrombosis. Chest. 1989. 95:498–502.
9. von Tempelhoff GF, Dietrich M, Niemann F, Schneider D, Hommel G, Heilmann L. Blood coagulation and thrombosis in patients with ovarian malignancy. Thromb Haemost. 1997. 77:456–461.
10. von Tempelhoff GF, Heilmann L, Hommel G, Schneider D, Niemann F, Zoller H. Hyperviscosity syndrome in patients with ovarian carcinoma. Cancer. 1998. 82:1104–1111.
11. De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2004. 50:187–196.
12. Rao LV. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992. 11:249–266.
13. Uno K, Homma S, Satoh T, Nakanishi K, Abe D, Matsumoto K, et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007. 96:290–295.
14. Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, et al. Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996. 60:412–417.
15. Recio FO, Piver MS, Hempling RE, Driscoll DL. Lack of improved survival plus increase in thromboembolic complications in patients with clear cell carcinoma of the ovary treated with platinum versus nonplatinum-based chemotherapy. Cancer. 1996. 78:2157–2163.
16. Rose SC, Zwiebel WJ, Nelson BD, Priest DL, Knighton RA, Brown JW, et al. Symptomatic lower extremity deep venous thrombosis: accuracy, limitations, and role of color duplex flow imaging in diagnosis. Radiology. 1990. 175:639–644.
17. Bradley MJ, Spencer PA, Alexander L, Milner GR. Colour flow mapping in the diagnosis of the calf deep vein thrombosis. Clin Radiol. 1993. 47:399–402.
18. Mattos MA, Londrey GL, Leutz DW, Hodgson KJ, Ramsey DE, Barkmeier LD, et al. Color-flow duplex scanning for the surveillance and diagnosis of acute deep venous thrombosis. J Vasc Surg. 1992. 15:366–375.
19. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004. 109:12 Suppl 1. I9–I14.
20. Carrier M, Le Gal G, Bates SM, Anderson DR, Wells PS. D-dimer testing is useful to exclude deep vein thrombosis in elderly outpatients. J Thromb Haemost. 2008. 6:1072–1076.
21. Moser KM, Fedullo PF, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994. 271:223–225.
22. Carrier M, Lee AY, Bates SM, Anderson DR, Wells PS. Accuracy and usefulness of a clinical prediction rule and D-dimer testing in excluding deep vein thrombosis in cancer patients. Thromb Res. 2008. 123:177–183.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download