Abstract
Sclerosing stromal tumor (SST) is a rare benign neoplasm of ovarian stromal origin and predominantly affects young women in the second and third decades. This tumor characteristically differentiates itself histologically and clinically from both thecomas and fibromas. We present a case of huge SST of the ovary weighing 10 kg in a 71-year-old postmenopausal woman with a brief review of the literature.
Sclerosing stromal tumor (SST) is a rare benign ovarian stromal tumor, first described by Chalvardjian and Scully in 1973.1 This tumor occurs predominantly in the second and third decades and is histologically characterized by the presence of pseudolobulation of cellular areas separated by edematous connective tissue, increased vascularity, and prominent areas of sclerosis.2-4 Most patients with this tumor present with menstrual irregularities and pelvic pain.
We present a case of huge SST of the ovary weighing 10 kg in a 71-year-old postmenopausal woman. This patient has atypical features, such as her postmenopausal age and huge mass size.
A 71-year-old gravida 5, para 5, multiparous woman presented to our institution in March 2008 with complaint of lower abdominal distension. At the time of the initial visit, the general condition of the patient appeared to be chronic ill looking, blood pressure was 130/90 mmHg, pulse was 84 beats/min and body temperature was 36.4℃. She had reached menopause at 50 years old. She was diagnosed as having diabetes mellitus upon hospitalization and management was started. On physical examination, the abdomen was severely distended. The patient complained of dyspnea and shortness of breath. On pelvic examination, total uterovaginal prolapse was noted. Transvaginal ultrasonography revealed the presence of a large mainly cystic mass. A computed tomography (CT) of the abdomen and the pelvis revealed a 43.9×28.3×34.7 cm complex cystic mass with peripheral enhancement (Fig. 1). There was no evidence of lymphadenopathy or metastatic cancer. Laboratory tests revealed a slightly rising serum CA 125 level (59.1 U/mL), whereas the routine blood tests and remaining tumor markers were within normal limits. The patient was diagnosed as having an ovarian tumor with a suspicion of sex-cord stromal tumor or primary ovarian cancer. Laparotomy was carried out in March 2008. Laparotomy findings showed a huge left ovarian tumor. The tumor was aspirated and the aspirated volume was 8000 cc. Adhesion among ovary, pelvic wall and bowel was noted. A subtotal abdominal hysterectomy with bilateral salpingo-oophorectomy and lysis of adhesion among ovary, pelvic wall and bowel were carried out. The fresh specimen was sent for the frozen section. The frozen section was reported as benign, suggestive of sclerosing stromal tumor. The patient's postoperative recovery was uneventful. The patient was discharged on postoperative day 11. She has been followed on an outpatient basis without specific findings.
On gross examination, the removed left ovarian mass was huge, measuring 45×33×27 cm and weighing 10 kg. The external surface was smooth but showed diffuse hyperemic appearance with a focal yellow discoloration (Fig. 2A). On cut section, it was partly multilocular cystic and partly solid with diffuse necrosis and multifocal hemorrhage. The inner content of the cysts varied from blood clots, dark brown serous fluid, yellow mucinous fluid, and to dark brown necrotic tissue. The viable solid part showed gray white homogenous fibrotic appearance (Fig. 2B).
Microscopically, the viable solid area of the tumor revealed a pseudolobular pattern in which cellular areas were separated by hypocellular areas of dense collagen tissue (Fig. 3A). The cellular areas were composed of an admixture of fibroblasts and round to oval lutenized theca like cells with moderate amounts of vacuolated cytoplasm and small, eccentrally located nucleoli are inconspicuous (Fig. 3B). The remaining parenchyma of the cystic area was diffusely necrotic without viable tissue.
The results of immunohistochemical stainings were positive for desmin (DAKO, Denmark, 1:500) and smooth muscle actin (Neomarker, USA, 1:1000) and negative for S-100 protein (DAKO, Denmark, 1:1000) (Fig. 3C, D).
Based on these histomorphologic findings, the diagnosis of sclerosing stromal tumor was made.
The vast majority of tumors in the thecoma-fibroma group are readily subcategorized based on relatively distinct clinical and histologic characteristics. The major subcategories include thecoma, fibroma-fibrosarcoma, and sclerosing stromal tumor (SST).2
SSTs were initially described by Chalvardjian and Scully in 1973 as a distinct subgroup within the thecoma-fibroma family of ovarian tumors. Accounting for less than 5% of sex cord-stromal tumors, this relatively rare tumor characteristically differentiates itself histologically and clinically from both thecomas and fibromas. Histologically, the presence of pseudolobulation of cellular areas separated by edematous connective tissue, increased vascularity, and prominent areas of sclerosis are distinguishing features. Clinically, SSTs tend to occur in the second and third decades of life, with a mean patient age of 28 years, whereas other types of stromal tumors are most common in the fifth and sixth decades. Most patients with SSTs present with menstrual irregularities and pelvic pain. Ascites may be seen but is rare; this contrasts SSTs from fibromas.2
Chalvardjian and Scully,1 who described the first ten cases, did not find convincing evidence of hormonal activity, as only half of their patients had abnormal uterine bleeding; however, other investigators have described the presence of steroid function in this type of neoplasia. Damjanov et al.5 reported a case in which urinary excretion of estrogens and androgens decreased after excision of the tumor. Postoperatively, their patient experienced resumption of normal cycles with ovulation, suggesting that the tumor was hormonally active and interfering with ovulation. Tsukamoto et al.6 reported a case in which anovulation and lipid staining characteristics suggested hormonal activity. Gee and Russell7 reported on 5 patients, 2 of who had suggestive evidence of estrogenic activity from the tumor.
To date, all SSTs have been clinically benign. Although a recent report noted an elevated CA 125 level, no specific tumor marker has been identified for SSTs to date.
Surgical removal of the tumor is curative, and there is no local or distant recurrence.
SSTs were shown at ultrasonography to be solid and cystic adnexal masses with centrally located, multiple, round or cleft-like cysts. Color Doppler ultrasonography of SSTs revealed prominent vascularity in the peripheral portion and central intercystic spaces.16 Magnetic resonance imaging (MRI) findings include a large mass with hyperintense cystic components or a heterogeneous solid mass of intermediate to high signal intensity on T2-weighted MRI. The thick peripheral hypointense rim on T2-weighted imaging is a compressed ovarian cortex due to a slow growing tumor. There is striking contrast enhancement with internal small cleft and cysts. On dynamic contrast enhanced images, the tumors reveal early peripheral enhancement with centripetal progression. Striking early enhancement reflects the cellular areas with their prominent vascular networks, and an area of prolonged enhancement in the inner portion of the mass represents the collagenous hypocellular area. These findings can be useful in differentiating SST from fibroma because fibroma shows absence of early enhancement and delayed accumulation of the contrast material.17,18
The differential diagnosis for SST includes subserosal leiomyoma, epithelial ovarian cancers and sex-cord stromal tumors. Typical parauterine leiomyomas show lower or similar signal intensity compared with normal myometrium on T1-weighted images, and shows lower signal intensity on T2-weighted images. Granulosa cell tumors are estrogen-secreting neoplasms. They are hemorrhagic, and sometimes flow voids can be seen near the mass. Thecomas and fibromas usually show extremely low signal intensity on T2-weighted images, though some exhibit hyperintense areas and weak enhancement on contrast MRI. Epithelial ovarian cancers are mostly seen in the postmenopausal period. Invasive growth and metastatic dissemination are important findings that distinguish epithelial ovarian cancers from benign ovarian tumors.19
Immunohistochemistry of desmin and smooth muscle actin is useful in distinguishing sclerosing stromal tumors from thecomas and fibromas. It is suggested that SST is derived from a population of muscle-specific actin-positive elements from the theca externa, namely the perifollicular myoid stromal cell.20
This case illustrates two atypical features, such as postmenopausal age and huge mass size in SST. So, We present a case of huge SST of the ovary weighed 10 kg in a 71-year-old postmenopausal woman with a brief review of the literature.
Figures and Tables
Fig. 1
Contrast-enhanced CT scans show a 43.9×28.3×34.7 cm cystic mass with peripheral enhancement. (A) Axial image. (B) Coronal image.
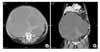
Fig. 2
Gross finding of left ovarian mass (45×33×27 cm). (A) The external surface is relatively smooth but shows diffuse hyperemic appearance with focal yellow discoloration. (B) On opening, it is a multilocular cystic mass with solid portion, showing diffuse necrotic and hemorrhagic, variegated appearance. The content varies from blood clots, dark brown serous fluid, yellow mucinous fluid, and to dark brown necrotic tissue.
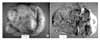
Fig. 3
Microscopic finding of viable area. (A) It shows an alternative pseudolobular pattern which consist of cellular and hypocellular areas (H&E, ×40). (B) The former consists of oval to round vaculated lutenized thecalike cells and fibroblasts. The latter consists of dense collagenous tissue (H&E, ×400). Immunohistochemistry shows positive for Desmin (C) and smooth muscle actin (D) (×400).

Table 1
A literature summary of the patients with SST of the ovary in Korea

R: right, L: left, RO: right oophorectomy, LO: left oophorectomy, RSO: right salpingo-oophorectomy, LSO: left salpingo-oophorectomy, BSO: bilateral salpingo-oophorectomy, TAH: total abdominal hysterectomy, BPLD: bilateral pelvic lymph node dissection, BEP: bleomycin, etoposide, cisplatin, NE: not evaluated, NS: no symptoms, WNL: within normal limits
References
1. Chalvadjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer. 1973. 31:664–670.
2. Hoskins WJ, Perez CA, Young RC, Barakat RR, Markman M, Randall ME. Principles and practice of gynecologic oncology. 2005. 4th ed. Philadelphia: Lippincott Williams & Wilkins;1016–1017.
3. Irving JA, McCluggage WG. Ovarian spindle cell lesions: A review with emphasis on recent developments and differential diagnosis. Adv Anat Pathol. 2007. 14:305–319.
4. Schneider DT, Jänig U, Calaminus G, Göbel U, Harms D. Ovarian sex cord-stromal tumors: A clinicopathological study of 72 cases from the Kiel Pediatric Tumor Registry. Virchows Arch. 2003. 443:549–560.
5. Damajanov I, Drobnjak P, Grizelj V, Longhino N. Sclerosing stromal tumor of the ovary: A hormonal and ultrastructural analysis. Obstet Gynecol. 1975. 45:675–679.
6. Tsukamoto N, Nakamura M, Ishikawa H. Case report: Sclerosing stromal tumor of the ovary. Gynecol Oncol. 1976. 4:335–339.
7. Gee DC, Russell P. Sclerosing stromal tumours of the ovary. Histopathology. 1979. 3:367–376.
8. Lee JH, Park SY, Lee KH, Cho EY. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 1988. 31:1129–1132.
9. Lee KJ, Park JH, Park CH. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 1990. 33:880–883.
10. Kim CN, Lee SK, Kim SB, Yang MH. Two cases of sclerosing stromal tumor of the ovary. Korean J Gynecol Oncol Colposcopy. 1994. 5:70–76.
11. Kim SB, Shin MC, Kim HG, Yun KJ, Moon HB. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 1994. 37:2302–2305.
12. Kim JC, Lee DW, Lee YH, Lee SW, Cho Y, Ro ES, et al. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 2002. 45:1431–1434.
13. Shin CS, Kim JJ, Yun CS, Cho HJ, Bae KH, Han KS, et al. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 2003. 46:1818–1822.
14. Yoon GS, Kim MS. A case of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 2003. 46:2052–2055.
15. Park HI, Kim JS, Choi SD, Sunwoo JG, Bae DH, Kim YH, et al. A case report of sclerosing stromal tumor of the ovary. Korean J Obstet Gynecol. 2004. 47:413–417.
16. Deval B, Rafii A, Darai E, Hugol D, Buy JN. Sclerosing stromal tumor of the ovary: Color Doppler findings. Ultrasound Obstet Gynecol. 2003. 22:531–534.
17. Jung SE, Rha SE, Lee JM, Park SY, Oh SN, Cho KS, et al. CT and MRI findings of sex cord-stromal tumor of the ovary. AJR Am J Roentgenol. 2005. 185:207–215.
18. Mikami M, Tanaka K, Komiyama S. Magnetic resonance imaging in sclerosing stromal tumor of the ovary. Int J Gynaecol Obstet. 2003. 83:319–321.
19. Tanaka YO, Nishida M, Yamaguchi M, Kohno K, Saida Y, Itai Y. MRI of gynaecological solid masses. Clin Radiol. 2000. 55:899–911.
20. Andrade LA, Gentilli AL, Polli G. Sclerosing stromal tumor in an accessory ovary. Gynecol Oncol. 2001. 81:318–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download