Abstract
Purpose
In the gastric mucosa of Helicobacter pylori (H. pylori)-infected patients with gastritis or adenocarcinoma, proliferation of gastric epithelial cells is increased. Hyperproliferation is related to induction of oncogenes, such as β-catenin and c-myc. Even though transcription factors NF-κB and AP-1 are activated in H. pylori-infected cells, whether NF-κB or AP-1 regulates the expression of β-catenein or c-myc in H. pylori-infected cells has not been clarified. The present study was undertaken to investigate whether H. pylori-induced activation of NF-κB and AP-1 mediates the expression of oncogenes and hyperproliferation of gastric epithelial cells.
Materials and Methods
Gastric epithelial AGS cells were transiently transfected with mutant genes for IκBα (MAD3) and c-Jun (TAM67) or treated with a specific NF-κB inhibitor caffeic acid phenethyl ester (CAPE) or a selective AP-1 inhibitor SR-11302 to suppress activation of NF-κB or AP-1, respecively. As reference cells, the control vector pcDNA was transfected to the cells. Wild-type cells or transfected cells were cultured with or without H. pylori.
Results
H. pylori induced activation of NF-κB and AP-1, cell proliferation, and expression of oncogenes (β-catenein, c-myc) in AGS cells, which was inhibited by transfection of MAD3 and TAM67. Wild-type cells and the cells transfected with pcDNA showed similar activities of NF-κB and AP-1, proliferation, and oncogene expression regardless of treatment with H. pylori. Both CAPE and SR-11302 inhibited cell proliferation and expression of oncogenes in H. pylori-infected cells.
Helicobacter pylori (H. pylori) infection is related to the development of gastritis, peptic ulcer, and gastric adenocarcinoma.1 A hallmark of H. pylori-associated gastric change is hyperproliferation of gastric epithelial cells.23 Oncogenes, such as β-catenin and c-myc, stimulate cell proliferation and promote malignant changes. H. pylori infection leads to an increase of nuclear β-catenin in gastric epithelial NCI-N87 cells.4 In the nucleus, β-catenin serves as a transcriptional regulator.56 c-Myc is one of target genes that are regulated by β-catenin.7 As a proto-oncogene, c-myc stimulates the expression of genes, which are involved in cell proliferation.8
Reactive oxygen species (ROS) are one of the potential toxic factors in H. pylori-induced gastric injury.9 The levels of ROS were increased in the gastric mucosa of H. pylori-infected patients.10 Previously, we and others showed that nicotinamide adenine dinucleotide phosphate oxidase produces ROS in H. pylori-infected gastric epithelial cells11 and gastric mucosa of humans and mice.1213 H. pylori-induced ROS production stimulates the expression of various genes by activating NF-κB and AP-1.14 Since these redox-sensitive transcription factors are activated by ROS,15 ROS may trigger the activation of these transcription factors in H. pylori-infected gastric epithelial cells. NF-κB regulates immune response, inflammatory reactions, cell proliferation, and apoptosis. IκB-α acts as the cytoplasmic inhibitory protein of NF-κB.16 Activation of NF-κB and AP-1 is shown in H. pylori-infected human gastric epithelial AGS cells.17 Nollet, et al.18 reported that NF-κB and AP-1 play a role in β-catenin expression in certain situations. However, whether NF-κB and AP-1 directly regulate the expression of β-catenin or c-myc in H. pylori-infected cells has not been clarified. The purpose of this study is to investigate whether H. pylori-induced activation of NF-κB and AP-1 mediates the expression of oncogenes (β-catenin, c-myc) and hyperproliferation of gastric epithelial cells.
A human gastric epithelial cell line AGS (adenocarcinoma gastric, ATCC CRL 1739) and H. pylori (strain NCTC 11637) were obtained from the American Type Culture Collection (Manassas, VA, USA). AGS cells were cultured as previously described.14 H. pylori was inoculated onto chocolate agar plates at 37℃ under microaerophilic conditions using GasPak™ EZ Gas Generating Pouch Systems (BD Biosciences, San Jose, CA, USA).14 Prior to infection, H. pylori were harvested and then suspended in antibiotic-free cell culture medium. H. pylori was added to cultured cells at a bacterium/cell ratio 50:1. In the ratio of bacterium/cell (50:1), H. pylori did not induce apoptotic cell death, which was reported in our previous study.19
A mutated IκBα gene, called MAD3 double-point mutant was prepared as described previously14 to inhibit activation of NF-κB. A dominant negative mutant of c-Jun, called TAM67, was a kind gift from Dr. Andreas von Knethen (University of Erlangen, Erlangen, Germany) and transfected to AGS cells to inhibit AP-1 activation. The control vector pcDNA (Invitrogen Corp., Carlsbad, CA, USA) was transfected to the cells instead of mutant genes for IκBα (MAD3) and c-Jun (TAM67). Subconfluent AGS cells were transfected with DOTAP {N-[1-(2,3-dioleoyloxy) propyl]-N,N,N trimethyl ammonium methylsulfate} (Boehringer-Mannheim, Pentzberg, Germany) for 16 h.11 The transfected cells were cultured with or without H. pylori.11 Wild-type cells were cultured with or without H. pylori and expressed as control and none cells. The cells transfected with pcDNA, MAD3, and TAM67 were expressed as pcDNA, MAD3, and TAM67 cells. In the other set of experiment, AGS cells were treated with a specific NF-κB inhibitors caffeic acid phenethyl ester (CAPE) (40 µM) (Sigma-Aldrich, St. Louis, MO, USA) or selective AP-1 inhibitor SR-11302 (2 µM) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h before H. pylori infection and cultured for 24 h (protein levels of oncogenes) and 48 h (viable cell numbers).
The cells were infected with H. pylori for 1 h (for NF-κB and AP-1), 24 h (for thymidine incorporation and oncogene expression), and for 72 h (viable cell numbers). The time points for determining NF-κB and AP-1, thymidine incorporation, oncogene expression, and viable cell numbers were adapted from a previous study.11
Viable cell numbers was determined by direct counting with a hemocytometer using the trypan blue exclusion test. For thymidine incorporation, 1 µCi/mL [3H] thymidine (Amersham Biosciences, Piscataway, NJ, USA) was added to the cells and cultured with or without H. pylori for 24 h.20 The cells were washed, incubated in 10% trichloroacetic acid and a solution consisting of 0.3 M NaOH and 1% sodium dodecyl sulfate (SDS) as described.20 The radioactivity of cell extracts was measured by liquid scintillation counting. The relative amount of [3H] thymidine incorporation, which reflected the extent of DNA synthesis, was expressed as a percentage of wild-type cells cultured without H. pylori (none).
Nuclear extracts were prepared for electrophoretic mobility shift assay.14 DNA binding activity of NF-κB or AP-1 was determined by the previously described method.14
For real-time PCR analysis, total RNA in cells were isolated and converted into cDNA by reverse transcription process using a random hexamer and virus reverse transcriptase (Promega, Madison, WI, USA). RNA expression levels of β-catenin, c-myc and β-actin were determined by the method described previously.11
For Western blot analysis, proteins in whole cell extracts were subjected to 8–12% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked using 3–5% nonfat dry milk in Tris-buffered saline and 0.2% Tween 20 (TBS-T), incubated with antibodies for β-catenin, c-myc, or actin (Santa Cruz Biotechnology, Dallas, TX, USA) diluted in TBS-T containing 3% dry milk, and washed with TBS-T. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies and visualized by the enhanced chemiluminescence detection system (Santa Cruz Biotechnology).11 To measure the density ratios among protein bands, the blots were scanned using a Bio-Rad laser densitometer (GS-700). The Scion image program (Scion Corporation, Frederick, MD, USA) was used to measure band intensities. The Western blot results presented in each figure are representative of four independent experiments. The protein level was compared to that of the loading control actin and expressed as the percentage ratio of the band densities.
One-way ANOVA and Newman-keul's test were used for determining the statistical differences. All values are expressed as mean±SE of four different experiments. A value of p<0.05 was considered statistically significant.
To determine the role of NF-κB and AP-1 in cell proliferation and oncogene expression, the cells were transiently transfected with mutant genes for IκBα (MAD3) and c-Jun (TAM67) and then cultured with H. pylori. As shown Fig. 1A, H. pylori-infection induced activation of NF-κB and AP-1 in wild-type cells (control) and the cells transfected with pcDNA (pcDNA) at 1 h-culture. H. pylori-induced activation of NF-κB and AP-1 were inhibited by transfection of MAD3 and TAM67. Similarly, H. pylori-stimulated cell proliferation time-dependently (Fig. 1B), and DNA synthesis at 24 h-culture (Fig. 1C) was inhibited by transfection with the mutants. However, transfection of MAD3 and TAM67 had no effect on the cells cultured without H. pylori. It may be explained that NF-κB and AP-1 were not activated in the cells cultured without H. pylori. As shown in Fig. 2, the mRNA and protein levels of β-catenin and c-myc were lower in the cells transfected with MAD3 and TAM67 than those transfected with pcDNA or wild-type cells cultured with H. pylori at 24 h-culture.
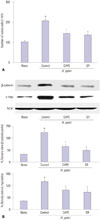
CAPE, one of the active compounds of propolis, has been shown to inhibit NF-κB activation.2122 SR-11302 is a retinoid that transrepresses AP-1 without transactivating the retinoic acid response element.2324 To investigate the involvement of NF-κB and AP-1 activities on H. pylori-induced oncogene expression and hyperproliferation, the cells were pretreated with CAPE or SR-11302 and then cultured with H. pylori. As shown in Fig. 3, CAPE and SR-11302 inhibited H. pylori-induced cell proliferation (determined by viable cell numbers at 48 h-culture) in AGS cells (Fig. 3A). H. pylori-induced expression of β-catenin and c-myc was inhibited by CAPE and SR-11302 in AGS cells at 24 h-culture (Fig. 3B). Taken together, H. pylori-induced oncogene expression and hyperproliferation are mediated by activation of NF-κB and AP-1 in gastric epithelial AGS cells.
In the present study, we found that activation of NF-κB and AP-1 transcriptionally regulate cell proliferation and expression of β-catenin and c-myc in H. pylori-infected gastric epithelial AGS cells. Hyper-proliferation of gastric epithelial cells and up-regulation of several gene expressions are reported to be associated with ROS under H. pylori infection. Even though ROS are considered to be responsible for the proliferation and oncogene expression, how ROS mediate cell proliferation in H. pylori-infected cells has not been clarified. We demonstrated that redox-sensitive transcription factors NF-κB and AP-1 mediate cell proliferation by inducing important oncogenes β-ca-tenin and c-myc in H. pylori-infected AGS cells. However, this study is limited in proving the hypothesis since AGS cells are already transformed cancer cells. Further study should be performed to establish the role of NF-κB and AP-1 on oncogene expression using primary gastric epithelial cells isolated from normal gastric tissues and in vivo animal models.
For the signaling mechanism for cell proliferation, β-catenin inactivates glycogen synthase kinase 3β and migrates to the nucleus, which induces the expression of cyclin D1. Bandapalli, et al.25 reported that overexpression of β-catenin increases its nuclear level and carcinogenesis including metastasis. In the present study, inhibition of NF-κB and AP-1 upon transfection of MAD3 or TAM67 suppressed the expression of β-catenin in H. pylori-infected cells. These results suggest that H. pylori may activate β-catenin through modulation of NF-κB and AP-1 activities in gastric epithelial cells. Even though c-myc expression is reported to be regulated by β-catenin,78 there has been no studies to determine the role of NF-κB and AP-1 on c-myc expression in gastric epithelial cells. In the present study, we found that NF-κB and AP-1 regulate the expression of both β-catenin and c-myc at the transcription level, which was determined using transfection of MAD3 (a mutated IκBα gene) or TAM67 (a dominant negative mutant of c-Jun) or treatment with CAPE (a specific NF-κB inhibitor) or SR-11302 (a selective AP-1 inhibitor). Therefore, targeting transcription factor NF-κB and AP-1 may be beneficial for preventing progression of H. pylori-associated carcinogenesis by suppressing expression of β-catenin and c-myc, as well as hyper-proliferation of gastric epithelial cells.
Figures and Tables
Fig. 1
Activation of NF-κB and AP-1, viable cell numbers, and thymidine incorporation of H. pylori-infected cells with or without transfection of MAD3 or TAM67. (A) The cells were cultured with H. pylori for 1 h. The DNA binding activities of NF-κB and AP-1 were determined by EMSA. (B) Viable cell numbers were determined by the trypan blue exclusion assay for indicated time period. *p<0.05 vs. 0 h, †p<0.05 vs. H. pylori (the cells without transfection of MAD3 and TAM67 and cultured with H. pylori) or H. pylori+pcDNA (the cells with transfection of pcDNA and cultured with H. pylori). (C) DNA synthesis was determined by thymidine incorporation. [3H] Thymidine was added to the cells and cultured with H. pylori for 24 h. *p<0.05 vs. corresponding none (the cells cultured without H. pylori), †p<0.05 vs. corresponding H. pylori (the cells without transfection of MAD3 and TAM67 and cultured with H. pylori) or H. pylori pcDNA (the cells with transfection of pcDNA and cultured with H. pylori). AGS, adenocarcinoma gastric; EMSA, lectrophoretic mobility shift assay; H. pylori, Helicobacter pylori.

Fig. 2
Expression of β-catenin and c-myc of H. pylori-infected AGS cells with or without transfection of MAD3 or TAM67. The cells were cultured in with or without H. pylori for 24 h. (A) mRNA expression of β-catenin and c-myc were measured by real-time PCR analysis. *p<0.05 vs. none (the cells cultured without H. pylori), †p<0.05 vs. H. pylori control (the cells without transfection of MAD3 and TAM67 and cultured in with H. pylori) or H. pylori pcDNA (the cells transfected with pcDNA and cultured with H. pylori). (B) Protein levels of β-catenin and c-myc were determined by Western blot analysis. Actin served as a loading control. The protein level was compared to that of the loading control actin and expressed as the percentage ratio of the band densities. All data are presented as the mean±SE of four independent experiments. *p<0.05 vs. none (the cells cultured without H. pylori), †p<0.05 vs. H. pylori control (the cells without transfection of MAD3 and TAM67 and cultured in with H. pylori) or H. pylori pcDNA (the cells transfected with pcDNA and cultured with H. pylori). H. pylori, Helicobacter pylori.

Fig. 3
Viable cell numbers and expression of β-catenin and c-myc of H. pylori-infected AGS cells with treatment of CAPE or SR-11302. (A) The cells were pretreated with CAPE or SR-11302 and cultured with H. pylori for 48 h. Viable cell numbers were determined by the trypan blue exclusion assay. *p<0.05 vs. none, †p<0.05 vs. H. pylori control (the cells without treatment of CAPE or SR-11302 and cultured with H. pylori). (B) Protein levels of β-catenin and c-myc were determined by Western blot analysis. Actin served as a loading control. The protein level was compared to that of the loading control actin and expressed as the percentage ratio of the band densities. All data are presented as the mean±SE of four independent experiments. *p<0.05 vs. none (the cells cultured without H. pylori), †p<0.05 vs. H. pylori control (the cells with H. pylori). H. pylori, Helicobacter pylori.
ACKNOWLEDGEMENTS
This study was supported by a grant from the NRF of Korea, funded by the Korean government (MSIP) (NRF-2012R1A1A2 043423).
References
1. Negrini R, Savio A, Poiesi C, Appelmelk BJ, Buffoli F, Paterlini A, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996; 111:655–665.

2. Nagy TA, Wroblewski LE, Wang D, Piazuelo MB, Delgado A, Romero-Gallo J, et al. β-Catenin and p120 mediate PPARδ-dependent proliferation induced by Helicobacter pylori in human and rodent epithelia. Gastroenterology. 2011; 141:553–564.

3. Peek RM Jr, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, et al. Helicobacter pylori cagA+strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997; 89:863–868.

4. Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer. 2010; 9:31.
5. Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998; 280:596–599.

6. Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996; 382:638–642.

7. Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000; 60:1671–1676.
8. Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999; 19:1–11.

9. Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Production of chemokines and reactive oxygen species by human neutrophils stimulated by Helicobacter pylori. Helicobacter. 2002; 7:170–174.

10. Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994; 35:179–185.

11. Byun E, Lim JW, Kim JM, Kim H. α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells. Mediators Inflamm. 2014; 2014:380830.
12. Keenan JI, Peterson RA 2nd, Hampton MB. NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic Biol Med. 2005; 38:1188–1196.
13. Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, et al. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007; 43:1627–1638.

14. Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004; 84:49–62.

15. Müller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997; 11:301–312.

16. Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002; 109:S81–S96.
17. Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003; 48:257–265.
18. Nollet F, Berx G, Molemans F, van Roy F. Genomic organization of the human beta-catenin gene (CTNNB1). Genomics. 1996; 32:413–424.

19. Lim JW, Kim H, Kim KH. NF-kappaB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Radic Biol Med. 2001; 31:355–366.

20. Coward L, Smith M, Kirk M, Barnes S. Chemical modification of isoflavones in soyfoods during cooking and processing. Am J Clin Nutr. 1998; 68:6 Suppl. 1486S–1491S.

21. Chen MF, Keng PC, Lin PY, Yang CT, Liao SK, Chen WC. Caffeic acid phenethyl ester decreases acute pneumonitis after irradiation in vitro and in vivo. BMC Cancer. 2005; 5:158.

22. Jung WK, Choi I, Lee DY, Yea SS, Choi YH, Kim MM, et al. Caffeic acid phenethyl ester protects mice from lethal endotoxin shock and inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW 264.7 macrophages via the p38/ERK and NF-kappaB pathways. Int J Biochem Cell Biol. 2008; 40:2572–2582.

23. Fanjul A, Dawson MI, Hobbs PD, Jong L, Cameron JF, Harlev E, et al. A new class of retinoids with selective inhibition of AP-1 inhibits proliferation. Nature. 1994; 372:107–111.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download