Abstract
Hemangioblastoma (HBL) in the pituitary stalk is extremely rare. Only 16 such cases have been reported in the past and 5 cases have been treated with surgical procedure. Here, we report surgical case of HBL in the pituitary stalk diagnosed in a 34-year-old woman. The patient underwent a gross-total resection via the modified lateral supra-orbital approach. No recurrence was observed in two years after surgery. To our knowledge, this is the 17th case of HBL in the pituitary stalk and the 6th surgical case. If the tumor is symptomatic and the volume is over 5 cubic centimeters as in our case, we recommend that the surgical resection of the HBL in the pituitary stalk is a more safe and reasonable than radiotherapy.
Hemangioblastoma (HBL), hypervascular-tumor of the central nervous system, accounts for 2% of all primary intracranial tumors and 10% of posterior fossa tumors.1,2 Cases of HBL in the pituitary stalk are exceptionally rare. To date, only 16 cases have been described.3,4,5,6,7,8,9,10,11 We report the 17th case of pituitary stalk HBL with a literature review focusing on appropriate treatment modalities and prognosis.
This retrospective study protocol was approved by the Hospital Institutional Review Board.
A 34-year-old female patient took medication because of her irregular menstrual cycles and amenorrhea started seven years ago. In endocrine examination, all the hormone levels were normal, except an increase of thyroid-stimulating hormone (20.3 ng/mL). An ophthalmological examination revealed slight decreases of visual acuity of both eyes (0.5) with normal visual field and ocular movement. Magnetic resonance imaging (MRI) revealed an intensely well-contrasted homogeneous solid mass with a size of 2.3×2×2.4-cm3 in sellar lesion, which was adjacent to the bilateral hypothalamus and optic tracts (Fig. 1). Hypervascular mass adjacent to both optic tracts were found in T2 weighted image. Based on the MRI findings, we suggested the following potential diagnoses: HBL, meningioma, pituitary adenoma, craniopharyngioma, and pituicytoma. Trans-femoral cerebral angiography showed the tumor fed by the right superior hypophyseal and the left posterior communicating (P-COM) and anterior choroidal arteries. Small feeders from bilateral ophthalamic arteries could not be superselected due to acute angulation and relatively small sizes. The feeders from the anterior choroidal artery were too small and dangerous to embolize. The feeders from the P-COM artery were relatively large, but the superselection could not be done. The right mainfeeder (superior hypophyseal artery) was superselected and embolized with 15% glue mixture (glue and lipiodol).
The operation was carried out through the modified lateral supra-orbital (MLSO) approach. The bone flap was made including the supraorbital bone, orbital roof, frontozygomatic process and frontal bone. And then the optic canal was unroofed with anterior clinoidectomy. The MLSO approach can provide sufficient operation field for suprasellar, parasellar, and retrosellar tumors. We have performed a number of tumor operations via this approach with good outcome since 1996. We did not choose the endonasal approach, because this approach is difficult to handle the profuse tumor bleeding. After the laminar terminalis was opened, the tumor was exposed. The tumor tissue was hyperemic and reddish (Fig. 2). It was very difficult to dissect and remove the tumor from the surrounding hypothalamus and optic chiasm because the tumor bleeding was too profuse to control. After the bleeding was controlled, we totally removed the tumor. The pituitary stalk should be sacrificed to totally remove the tumor. The patient's postoperative clinical course was uneventful. In pathologic examination, prominent vascular channels with foamy vacuolated stromas were observed. Neither mitoses nor necrosis were observed. Immunohistochemical tests using inhibin and S-100 showed positive findings. We finally diagnosed it as HBL. In post-operative MRI, the tumor was found to be totally removed (Fig. 1). The visual acuity of the right eye was 0.1 and that of the left eye was 0.3 with visual field defect. Diabetes insipidus occurred. Other pituitary hormones were at normal levels. In a follow-up examination after 24 months, there was no recurrence and the right visual acuity has improved from 0.1 to 0.5. No additional radiation therapy (RT) was conducted. Several examinations were conducted to see whether there were tumors in other organs after a definite pathological diagnosis. Abdominal computed tomography (CT) showed an about 4.2 cm-sized benign teratoma in the left ovary.
HBL occurs typically in the cerebellum, brainstem, and spinal cord. Supratentorial HBLs are sporadic tumors, which are relatively rare.12 Sixteen cases of HBL in the pituitary stalk have been reported, among which, eight patients had no symptoms, therefore, they were not treated.3,4,5,6,7 Of the eight cases that had symptoms, five patients were operated, among whom, four patients had a GTR. The previously reported cases of HBL in the pituitary stalk are summarized in Table 1.
When there is a mass in the pituitary stalk, differential diagnosis is difficult. The following can be used to diagnosis: 1) HBL does not have a dural tail, one of the attributes of meningioma; 2) It is easier to contrast HBL than a pituitary tumor; 3) Calcification, a good clue for a diagnosis of craniopharyngioma, is not present in HBL. We thought that our case might be pituicytoma, which might occur in the pituitary. Therefore, it would be necessary to discern hemangiopericytoma as well, since it can be well contrasted in T1 enhanced MRI.
Supratentorial HBLs have been reported to comprise only 1 to 6% of all HBLs associated with von Hippel-Lindau (VHL).11,13,14 In our case, an abdominal CT scan showed a teratoma in the left ovary. Accordingly, we recommended the genetic test to rule out the VHL. However, the patient refused to take such test.
Total resection might be the most important aspect in the treatment of HBL. In a series of studies by Mills, et al.,12 only GTR had an excellent regulation rate of tumor when there was no cystic component in the tumor. For a partial resection during the operation, it would be necessary to verify the recurrence with a long-term observation.
RT protocol for HBL has not been fully established.4 As for an analysis of fractionated RT as a therapeutic modality for supratentorial HBL, Mills, et al.12 reported that RT had a lower progression-free survival rate than GTR. Stereotactic radiosurgery can be effective for those tumors located in areas where it would be difficult to perform a surgical resection, because of a few side effects with a high suppression rate of tumor.15,16
It is important to totally remove HBL in the pituitary stalk without any complications, such as panhypopituitarism and visual disturbance, which might be why only 6 cases out of 17 cases were operated. Of the 6 surgical cases, five patients had a panhypopituitarism, and two patients had visual disturbance postoperatively. In this case, the pituitary stalk was sacrificed, but pituitary hormones were at normal levels. We thought that the reason is the migration of neuro-secretory material down the tract. When the stalk is severed by hypophysectomy or compressed by pressure from a tumor, the neuro-secretory material piles up on the proximal side of the cut and disappears distal to the lesion.17 Despite post-operative surgical complications, GTR might be the best choice for treating HBLs in the pituitary stalk, because GTR showed a good outcome for sporadic HBLs when a surgical resection was completed, whereas RT was not specifically proven to be effective in suppressing recurrence.4 The optimal timing and strategy of surgery HBLs in the pituitary stalk has not been determined. Lonser, et al.10 reported that asymptomatic HBLs in the pituitary stalk could be managed conservatively with observation on serial imaging, and endocrine and visual assessment. However, for symptomatic HBLs in the pituitary stalk, surgery or radiosurgery should be considered. The tumor volume was 2.3×2×2.4-cm3 in our case. Thus, surgical resection was chosen rather than radiosurgery because it is more safe and reasonable treatment in order to preserve the vision. If the tumor is symptomatic and the tumor volume is over 5 cubic centimeters as in our case, we recommend the surgical resection because it is more safe and reasonable to treat the HBL in the pituitary stalk than RT.
Figures and Tables
Fig. 1
MRI image of coronal and sagittal plane before and after operation. (A and D) MRI T1 image of coronal and sagittal plane before the operation, showing isointense and homogeneous suprasellar lesion. (B and E) T1 enhanced image showing that the tumor was adjacent to both optic tracts. (C and F) MRI T1 image of coronal and sagittal plane after the operation, showing the tumor was totally removed.
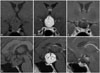
Fig. 2
Images of tumor during and after the gross-total resection. (A) Optic nerve (white arrow) and internal carotid artery (black arrow). (B) After laminar terminalis was opened, the tumor was seen. (C) The tumor tissue was hyperemic and reddish (white arrow). (D) The tumor was totally removed.
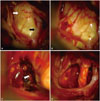
Table 1
Cases of Hemangioblastoma in the Pituitary Stalk

| Author | Case/age/sex | Sign/symptom | Preoperative endocrine function | VHL | Resection | Follow-up |
|---|---|---|---|---|---|---|
| Grisoli, et al.7 | 1/28/F | Headache, galatorrhea, obesity, alopecia | Elevated prolactin in TRH stimulation (14–27 ng/mL) | - | Total | Post operative panhypopituitarism |
| Neumann, et al.2 | 1/35/F | Headache, amenorrhea, DI | NA | - | NA | NA |
| Kouri, et al.8 | 1/20/F | Amenorrhea, DI | Panhypopituitarism | + | Total | Stable panhypopituitarism |
| Goto, et al.6 | 1/33/F | Irregular menstruation | Slightly decrease in the response of LH & FSH to the GnRH stimulation test | + | Partial | Normal pituitary function |
| Fomekong, et al.4 | 1/51/F | Rt. inferior temporal quadrantanopia | Elevated prolactin (80.7 ng/mL) | + | Total | Post operative panhypopituitarism recovery of visual loss |
| Lonser, et al.10 | 8/UK/UK | Normal | + | No operation | NA | |
| Cao, et al.3 | 1/28/F | Galactorrhea, alopecia | Elevated prolactin (28.35 ng/mL) | + | No operation | NA |
| Fu, et al.5 | 1/49/M | Headache, polydipsia | NA | - | Total | Post operative panhypopituitarism |
| Lee, et al.9 | 1/34/M | Headache, dizziness diplopia | NA | + | No operation | NA |
| Our case | 1/36/F | Amenorrhea, galactorrhea | Elevated TSH (20.3 ng/mL) | + | Total | Visual disturbance |
References
1. Ho YS, Plets C, Goffin J, Dom R. Hemangioblastoma of the lateral ventricle. Surg Neurol. 1990; 33:407–412.

2. Neumann HP, Bender BU, Berger DP, Laubenberger J, Schultze-Seemann W, Wetterauer U, et al. Prevalence, morphology and biology of renal cell carcinoma in von Hippel-Lindau disease compared to sporadic renal cell carcinoma. J Urol. 1998; 160:1248–1254.

3. Cao Y, Gao P, Wang S, Zhao J. Pituitary infundibulum hemangioblastoma detected by dynamic enhancement MRI. Can J Neurol Sci. 2010; 37:697–699.

4. Fomekong E, Hernalsteen D, Godfraind C, D'Haens J, Raftopoulos C. Pituitary stalk hemangioblastoma: the fourth case report and review of the literature. Clin Neurol Neurosurg. 2007; 109:292–298.

5. Fu H, Hao S, Wu Z, Zhang J, Zhang L. Sporadic pituitary stalk hemangioblastoma. Neurol India. 2011; 59:937–938.

6. Goto T, Nishi T, Kunitoku N, Yamamoto K, Kitamura I, Takeshima H, et al. Suprasellar hemangioblastoma in a patient with von Hippel-Lindau disease confirmed by germline mutation study: case report and review of the literature. Surg Neurol. 2001; 56:22–26.

7. Grisoli F, Gambarelli D, Raybaud C, Guibout M, Leclercq T. Suprasellar hemangioblastoma. Surg Neurol. 1984; 22:257–262.

8. Kouri JG, Chen MY, Watson JC, Oldfield EH. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000; 92:1028–1035.

9. Lee KM, Kim EJ, Choi WS, Kim TS. Pituitary stalk hemangioblastoma in a von hippel-lindau patient : clinical course follow-up over a 20-year period. J Korean Neurosurg Soc. 2013; 53:297–299.

10. Lonser RR, Butman JA, Kiringoda R, Song D, Oldfield EH. Pituitary stalk hemangioblastomas in von Hippel-Lindau disease. J Neurosurg. 2009; 110:350–353.

11. Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, et al. Central nervous system lesions in von Hippel-Lindau syndrome. J Neurol Neurosurg Psychiatry. 1992; 55:898–901.

12. Mills SA, Oh MC, Rutkowski MJ, Sughrue ME, Barani IJ, Parsa AT. Supratentorial hemangioblastoma: clinical features, prognosis, and predictive value of location for von Hippel-Lindau disease. Neuro Oncol. 2012; 14:1097–1104.

13. Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003; 361:2059–2067.

14. Peyre M, David P, Van Effenterre R, François P, Thys M, Emery E, et al. Natural history of supratentorial hemangioblastomas in von Hippel-Lindau disease. Neurosurgery. 2010; 67:577–587.

15. Kano H, Niranjan A, Mongia S, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008; 63:443–450.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download