Abstract
Purpose
To evaluate the accuracy of postoperative refractive outcomes of combined phacovitrectomy for epiretinal membrane (ERM) in comparison to cataract surgery alone.
Materials and Methods
Thirty-nine eyes that underwent combined phacovitrectomy with intraocular lens (IOL) implantation for cataract and ERM (combined surgery group) and 39 eyes that received phacoemulsification for cataract (control group) were analyzed, retrospectively. The predicted preoperative refractive aim was compared with the results of postoperative refraction.
Results
In the combined surgery group, refractive prediction error by A-scan and IOLMaster were -0.305±0.717 diopters (D) and -0.356±0.639 D, respectively, compared to 0.215±0.541 and 0.077±0.529 in the control group, showing significantly more myopic change compared to the control group (p=0.001 and p=0.002, respectively). Within each group, there was no statistically significant difference in refractive prediction error between A-scan and IOLMaster (all p>0.05). IOL power calculation using adjusted A-scan measurement of axial length based on the macular thickness of the normal contralateral eye still resulted in significant postoperative refractive error (all p<0.05). Postoperative refraction calculated with adjusted axial length based on actual postoperative central foveal thickness change showed the closest value to the actual postoperative achieved refraction (p=0.599).
Conclusion
Combined phacovitrectomy for ERM resulted in significantly more myopic shift of postoperative refraction, compared to the cataract surgery alone, for both A-scan and IOLMaster. To improve the accuracy of IOL power estimation in eyes with cataract and ERM, sequential surgery for ERM and cataract may need to be considered.
Pars plana vitrectomy (PPV) combined with cataract surgery are usually performed in eyes with vitreoretinal disease and coexisting cataract, particularly when a cataract prevents adequate visualization of the retina. It is a safe and effective way to treat vitreoretinal pathology and cataracts simultaneously, and its functional outcomes are reported to be comparable to those of sequential surgery.1,2,3 In order to achieve a favorable postoperative visual outcome, accurate preoperative intraocular lens (IOL) power calculation is crucial, and precise measurement of axial length (AL) is the most important element in obtaining accurate estimations of the IOL calculation.4,5 However, some studies have reported that the refractive results after combined phacovitrectomy show a postoperative myopic shift, compared to predicted refraction obtained by either A-scan ultrasonography or optical biometry using IOLMaster.6,7,8,9,10,11,12 Nevertheless, only a few studies have compared postoperative refractive prediction error by A-scan and IOLMaster simultaneously, and no studies have used spectral domain OCT (SD-OCT) to measure the distance from the internal limiting membrane (ILM) to the retinal pigment epithelium (RPE) in patients with epiretinal membrane (ERM) in association with biometry.
In this study, we evaluated whether performing PPV with removal of the ERM combined with cataract surgery affects postoperative refractive prediction error with both A-scan and IOLMaster in comparison cataract surgery alone, and we also attempted to outline factors influencing refractive outcomes. Furthermore, we evaluated whether adjusted A-scan measured AL method based on macular thickness of the normal contralateral eye could minimize refractive prediction error.
This retrospective case control study was performed in 39 eyes of 39 patients who underwent combined phacoemulsification and vitrectomy for idiopathic epiretinal membranes (combined surgery group) and 39 eyes of 39 patients who received phacoemulsification for cataract only (control group) at Gangnam Severance Hospital from July 2008 to January 2010. Patients with diabetes, with any retinal vascular disorder, or with macular degeneration were excluded. A-scan ultrasonography (UD-6000, TOMEY, Nagoya, Japan) and IOLMaster (Carl Zeiss Meditec, CA, USA) were used simultaneously for preoperative AL measurement and IOL calculation using the SRK/T formulae. Informed consent was obtained from all patients, and the study was approved by the Institutional Review Boards at the Gangnam Severance Hospital. The study was performed in accordance with the tenets of the Declaration of Helsinki and all federal laws.
Extracapsular cataract extraction with phacoemulsification (2.8-mm clear corneal incision at the superior limbus) and posterior capsular IOL implantation with an acrylic foldable IOL were performed in all patients. The preoperative IOL power was targeted for emmetropia. The combined surgery group comprised patients who had undergone phacoemulsification and PPV due to an ERM without other retinal pathology influencing the macula, and cataract surgery was performed before vitrectomy. Vitreoretinal procedures included a 23-gauge PPV and removal of epiretinal membranes. Indocyanine green-assisted ILM peeling was performed in all cases. No gas was used in any eyes during the surgery. The sclerotomy sites were left without any sutures. All patients were treated by a single vitreoretinal surgeon (SSK).
Clinical examinations were performed preoperatively and at 3 months postoperatively. The examinations included manifest refraction, autokeratometry (K) using a ref-keratometer (RK-3; CANON Inc., Kanagawa, Japan), measurement of anterior chamber depth (ACD), AL using an A-scan and IOLMaster, and central foveal thickness (CFT) by SD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA). A-scan biometry was obtained using the applanation method. Postoperative refractive prediction error was calculated by subtracting the spherical equivalent (SE) of the actual refraction from the SE of the predicted refraction.
A-scan ultrasonography measured the distance from cornea to the ILM, while IOLMaster measured the distance to the RPE. CFT by SD-OCT was defined as the distance between the ILM and the RPE at the fovea.
Chi-squared test and independent Student t-tests were performed to assess differences in patient demographic data between groups. Independent t-test was used to assess differences in postoperative refractive prediction errors between groups. Paired t-test was used to evaluate differences in refractive prediction error between A-scan and IOLMaster within each group. Statistical analysis was conducted using SPSS software (18.0, SPSS Inc., Chicago, IL, USA). In all cases, a p-value less than 0.05 was considered statistically significant.
Baseline patient demographic data are shown in Table 1. Seventy-eight eyes of 78 patients were enrolled in the study. There was no significant difference between the combined surgery group and the control group regarding gender, age, preoperative spherical equivalents, keratometric value, ACD, and axial length. The axial length measured by IOLMaster was longer than that of the A-scan in both groups (p<0.0001) (Table 1). In the combined surgery group, refractive prediction error by A-scan and IOLMaster were -0.305±0.717 diopters (D) and -0.356±0.639 D, respectively, compared to 0.215±0.541 and 0.077±0.529 in the control group, respectively, revealing significantly more myopic change in the combined surgery group (p=0.001 and p=0.002, respectively) (Table 2). However, within each group, there was no statistically significant difference in refractive prediction error between A-scan and IOLMaster measurements (all p>0.05).
Table 3 shows postoperative changes of visual acuity, macular thickness, and refraction with IOL calculation by A-scan before and after combined surgery. The mean planned target refraction was -0.269±0.656 D, while the mean actual postoperative refractive outcome was -0.574±0.784 (p=0.012), showing a significant myopic shift. When the preoperative planned target refraction was recalculated with an adjusted AL obtained by using the CFT value of the healthy contralateral eye (preoperative CFT-normal contralateral eye CFT), the target refraction of the implanted IOL was -0.928±0.679 D, still showing a statistically significant difference from the achieved refraction (mean refractive prediction error of 0.311±0.687 D, p=0.014). The estimated IOL power was recalculated with an adjusted AL by adding the amount of actual postoperative CFT change (preoperative CFT-postoperative CFT using SD-OCT) to AL measured by A-scan, and the planned target refraction became -0.651±0.706 D, showing no statistically significant difference between the planned and achieved refractions (p=0.599).
In the combined surgery group, there was a statistically significant increase of both K values and ACD measured by IOLMaster, postoperatively (p=0.025 and p<0.0001, respectively). However, AL measured with IOLMaster did not show any significant difference between preoperative and postoperative values (p=0.338) (Table 3).
During the follow-up period, there were no cases with significant postoperative complications, such as cystoid macular edema, endophthalmitis, or hypotony.
Our study showed that the combined phacovitrectomy results in a significantly more myopic shift of postoperative refraction, compared to that of the control group using both A-scan and IOLMaster. Adjusted A-scan measured AL method based on the healthy contralateral eye still resulted in significant refractive prediction error; meanwhile, postoperative refraction calculated with adjusted AL based on actual postoperative CFT change showed the closest value to the actual postoperative achieved refraction.
Combined phacovitrectomy has become a common procedure as a result of recent progress in cataract and vitrectomy surgery. However, in combined phacovitrectomy, postoperative refractive errors are thought to arise from concomitant macular pathology, such as macular thickening due to epiretinal membrane. Kovács, et al.6 suggested that myopic shift results from underestimation of the axial length due to a thicker macula using A-scan ultrasonography. In contrast, Manvikar, et al.13 reported that there is no tendency toward a myopic shift in IOL power estimation using IOLMaster, while Falkner-Radler, et al.12 reported that there was myopic refractive change after combined phacovitrectomy despite IOL power calculation with IOLMaster. These studies suggested thicker retina preoperatively or anterior displacement of the IOL by the complete gas fill achieved after phacovitrectomy as potential mechanisms of myopic shift.6,7,8,9,11,12
A-scan ultrasound measures the distance between the anterior surface of the cornea and the ILM, whereas the IOLMaster measures the distance between the anterior corneal surface and the RPE and then calibrates to match the measurement of the A-scan. Verhulst and Vrijghem14 reported that the mean difference in axial length between ultrasound and optical biometry was 0.2 mm, and IOLMaster resulted in measurement of a longer axial length. These findings were consistent with our results in that the axial length measured by IOLMaster was longer than that of A-scan in both groups (p<0.0001) (Table 1). Thus, we hypothesized that an error in measurement of axial length due to the ERM could cause myopic shift in IOL calculation with A-scan. This is consistent with a previous finding of a postoperative myopic shift due to an error in measuring axial length in combined surgery.6
IOLMaster measures axial length to the RPE and thus should not be affected by retinal thickening due to the presence of ERM. However, our results showed that there was a similar degree of refractive prediction error obtained when using IOLMaster, compared to A-scan. Studies of IOL positioning reported that during the first postoperative week, IOL moves slightly forward, which is neutralized by a slight backward movement within 3 months.15 Falkner-Radler, et al.12 reported that, compared with patients in the cataract surgery group, patients in the combined surgery group had a mean postoperative myopic shift in refractive error and deeper ACD in IOL calculation with IOLMaster. The combined surgery group in our study also showed increased ACD postoperatively (p<0.0001) (Table 3).
A few studies have suggested that myopic shift might be the result of a postoperative increase in axial length caused by sclera thinning or stretching in or around the sclerotomy sites after vitrectomy.8,12 In our study, there was no significant change in axial length measured by IOLMaster after combined surgery (p=0.338) (Table 3). Therefore, it would be difficult to consider postoperative increases in axial length as a potential cause for a myopic shift following phacoemulsification and PPV in IOL power estimation with IOLMaster. Falkner-Radler, et al.12 speculated that the myopic shift by the IOLMaster calculation might result from a significantly higher postoperative K2 value than the baseline K2 value in the combined surgery group. This result was in close agreement with those of our study (Table 3); therefore, it is possible that a change in K value after surgery may also contribute to the postoperative refractive prediction error upon use of IOLMaster. There were also studies reporting that after PPV, vitreous gel is replaced by the aqueous after PPV, and refractive index decreases slightly, thereby resulting in a myopic shift.16,17,18 Theoretically, the myopic shift in vitrectomized eyes could be up to -0.5 D.17 Considering that the mean myopic change was -0.36 D in the IOL power calculation with IOLMaster, we speculate that vitrectomy itself could also play a role in causing myopic change after phacovitrectomy. Currently, there are no formulas available for vitrectomized eyes or phacovitrectomy, and a new generation of formulas may need to be developed in the future.19
In some IOL implantation cases with macular diseases, a double peak was observed by IOLMaster in axial length measurements, with the posterior peak of a double peak representing RPE.20 The report suggested that the anterior peak of the double peak may represent reflection from the surface of the retina, such as an ERM, an inner limiting membrane, or a posterior vitreous membrane. We speculated that this may also be a possible cause of severe myopic shift; however, in our study, there was only one patient in the combined surgery group who showed a double peak in the IOLMaster signal, and the anterior peak of the double peak was initially used to measure axial length. The refractive prediction error caused by this measurement of axial length was -1.6 D. When the measurement was corrected to use the posterior peak of the double peak, the refractive prediction error decreased to -0.8 D.
Since the use of small gauge sutureless incisions is known to reduce surgically induced astigmatism and phacovitrectomy was found to cause no significant postoperative astigmatic change,19,21 we can assume that performing sutureless vitrectomy combined with phacoemulsification would not cause significant astigmatic change.
There have been many attempts to reduce postoperative refractive prediction error after performing combined cataract surgery and PPV. A study conducted by Patel, et al.9 suggested that slight residual hyperopia could compensate for the myopic overcorrection. Kovács, et al.6 attempted to determine the adjusted planned ametropia by substituting the full axial length and the implanted IOL power into the SRK/T formula (12 eyes studied). This full axial length was determined in each case by adding the difference between the normal value of macular thickness and results of the OCT scans to the AL of the A-scan. Similarly, Sun and Choi22 proposed a correlation between postoperative refraction and the calculated predicted refraction using an adjusted AL with macular thickness change based on the contralateral eye (23 eyes studied). To apply this calculation prospectively to patients with increased macular thickness planning to undergo combined phacovitrectomy, they subtracted the macular thickness of the contralateral normal eye from the macular thickness of the affected eye, and then added this number to the A-scan measured AL. Although these various attempts to compensate for the myopic overcorrection using adjusted A-scan measured AL have proven partially effective in compensating for myopic shift, macular thickness in many patients does not decrease completely to a normal value after surgery, and each patient shows a different postoperative CFT change. For this reason, our analysis showed persistent postoperative refractive prediction error even when calculated with the adjusted AL, based on the difference in macular thickness from the healthy contralateral eye (p=0.014) (Table 3). For the most accurate assessment of CFT and AL with which to maximize postoperative refractive outcomes, it might prove to be better to perform vitrectomy first and then carry out cataract surgery based on the IOL calculation obtained after vitrectomy, in a sequential manner.
Many attempts to correct for myopic shift after combined phacovitrectomy have been based on IOL calculation using A-scan measurements of AL. As the cause of myopic shift in IOL power estimation using IOLMaster seemed to be multifactorial and unpredictable, there are limitations in attempts to compensate for the postoperative refractive prediction error by IOLMaster. Sequence of surgery, as well as time between surgeries, may need to be taken into consideration to improve refractive outcomes in cases under consideration for combined phacovitrectomy.19
There are a few limitations to this study. It is a retrospective case control study, and a prospective study with more patients would be needed in the future. Also, further studies comparing the refractive outcomes of combined phacovitrectomy versus sequential surgery are warranted in the future to optimize refractive outcomes. The use of the SRK/T formula rather than the Holladay and Hoffer Q formulas may also have contributed to refractive prediction error in addition to axial length.
In conclusion, combined phacovitrectomy resulted in significantly more myopic shift of postoperative refraction, compared to that of the control group, on both A-scan and IOLMaster. Myopic shift in IOL calculations with A-scan may be attributable to an underestimation of axial length due to ERM, while myopic shift in IOL power estimation using IOLMaster may be attributed to a combination of changes in K values, ACD, and refractive index due to removal of vitreous gel. Adjusted A-scan measured AL method based on a healthy contralateral eye still resulted in significant refractive prediction error, while postoperative refraction calculated with an adjusted AL based on actual postoperative CFT change showed the closest value to the actual postoperative achieved refraction. Therefore, to improve the accuracy of IOL power estimation in eyes with cataract and ERM, sequential surgery for ERM and cataract may need to be considered.
Figures and Tables
Table 1
Patient Demographic Data
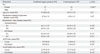
IOL, intraocular lens; ACD, anterior chamber depth.
*Chi-squared test was used for statistical analysis.
†Independent t-test was used for statistical analysis.
‡Combined surgery group=pars plana vitrectomy, removal of epiretinal membrane, and phacoemulsification and intraocular lens implantation.
§Control group=phacoemulsification and intraocular lens implantation only.
Table 2
Comparison of Refractive Prediction Error between the Combined Surgery Group and the Control Group

| Refractive prediction error (D)‡ | Combined surgery group (n=39) | Control group (n=39) | p value* |
|---|---|---|---|
| A-scan | -0.305±0.717 | 0.215±0.541 | 0.001 |
| IOLMaster | -0.356±0.639 | 0.077±0.529 | 0.002 |
| p value† | 0.616 | 0.073 |
Table 3
Postoperative Changes of Visual Acuity, Macular Thickness, and Refraction with IOL Calculation by A-Scan and Keratometry (K) Value, Anterior Chamber Depth (ACD), and Axial Length Measured by IOLMaster in the Combined Surgery Group

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine (Seoul, Korea) for 2012 (3-2012-0137).
References
1. Demetriades AM, Gottsch JD, Thomsen R, Azab A, Stark WJ, Campochiaro PA, et al. Combined phacoemulsification, intraocular lens implantation, and vitrectomy for eyes with coexisting cataract and vitreoretinal pathology. Am J Ophthalmol. 2003; 135:291–296.

2. Hwang JU, Yoon YH, Kim DS, Kim JG. Combined phacoemulsification, foldable intraocular lens implantation, and 25-gauge transconjunctival sutureless vitrectomy. J Cataract Refract Surg. 2006; 32:727–731.

3. Heiligenhaus A, Holtkamp A, Koch J, Schilling H, Bornfeld N, Lösche CC, et al. Combined phacoemulsification and pars plana vitrectomy: clear corneal versus scleral incisions: prospective randomized multicenter study. J Cataract Refract Surg. 2003; 29:1106–1112.

4. McEwan JR, Massengill RK, Friedel SD. Effect of keratometer and axial length measurement errors on primary implant power calculations. J Cataract Refract Surg. 1990; 16:61–70.

5. Olsen T. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 1992; 18:125–129.

6. Kovács I, Ferencz M, Nemes J, Somfai G, Salacz G, Récsán Z. Intraocular lens power calculation for combined cataract surgery, vitrectomy and peeling of epiretinal membranes for macular oedema. Acta Ophthalmol Scand. 2007; 85:88–91.

7. Suzuki Y, Sakuraba T, Mizutani H, Matsuhashi H, Nakazawa M. Postoperative refractive error after simultaneous vitrectomy and cataract surgery. Ophthalmic Surg Lasers. 2000; 31:271–275.

8. Jeoung JW, Chung H, Yu HG. Factors influencing refractive outcomes after combined phacoemulsification and pars plana vitrectomy: results of a prospective study. J Cataract Refract Surg. 2007; 33:108–114.

9. Patel D, Rahman R, Kumarasamy M. Accuracy of intraocular lens power estimation in eyes having phacovitrectomy for macular holes. J Cataract Refract Surg. 2007; 33:1760–1762.

10. Hotta K, Sugitani A. Refractive changes in silicone oil-filled pseudophakic eyes. Retina. 2005; 25:167–170.

11. Iwase T, Sugiyama K. Investigation of the stability of one-piece acrylic intraocular lenses in cataract surgery and in combined vitrectomy surgery. Br J Ophthalmol. 2006; 90:1519–1523.

12. Falkner-Radler CI, Benesch T, Binder S. Accuracy of preoperative biometry in vitrectomy combined with cataract surgery for patients with epiretinal membranes and macular holes: results of a prospective controlled clinical trial. J Cataract Refract Surg. 2008; 34:1754–1760.

13. Manvikar SR, Allen D, Steel DH. Optical biometry in combined phacovitrectomy. J Cataract Refract Surg. 2009; 35:64–69.

14. Verhulst E, Vrijghem JC. Accuracy of intraocular lens power calculations using the Zeiss IOL master. A prospective study. Bull Soc Belge Ophtalmol. 2001; 61–65.
15. Petternel V, Menapace R, Findl O, Kiss B, Wirtitsch M, Rainer G, et al. Effect of optic edge design and haptic angulation on postoperative intraocular lens position change. J Cataract Refract Surg. 2004; 30:52–57.

16. Gao Q, Chen X, Ge J, Liu Y, Jiang Z, Lin Z, et al. Refractive shifts in four selected artificial vitreous substitutes based on Gullstrand-Emsley and Liou-Brennan schematic eyes. Invest Ophthalmol Vis Sci. 2009; 50:3529–3534.

17. Mehdizadeh M, Nowroozzadeh MH. Postoperative induced myopia in patients with combined vitrectomy and cataract surgery. J Cataract Refract Surg. 2009; 35:798–799.

18. Byrne S, Ng J, Hildreth A, Danjoux JP, Steel DH. Refractive change following pseudophakic vitrectomy. BMC Ophthalmol. 2008; 8:19.

19. Hamoudi H, La Cour M. Refractive changes after vitrectomy and phacovitrectomy for macular hole and epiretinal membrane. J Cataract Refract Surg. 2013; 39:942–947.

20. Kojima T, Tamaoki A, Yoshida N, Kaga T, Suto C, Ichikawa K. Evaluation of axial length measurement of the eye using partial coherence interferometry and ultrasound in cases of macular disease. Ophthalmology. 2010; 117:1750–1754.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download