Abstract
Purpose
The aim of this study was to investigate the prognosis of papillary thyroid carcinoma (PTC) patients according to different pathologic grades of lymphocytic thyroiditis (LT).
Materials and Methods
This study included 144 PTC patients who underwent total thyroidectomy with radioactive iodine remnant ablation therapy. Pathologic grades of LT were separated at two points, chronic lymphocytic thyroiditis (CLT) and Hashimoto thyroiditis (HT). Patients were divided into two groupings according to the presence of the diseases (Grouping 1; patients with CLT or HT and without CLT or HT, Grouping 2; patients with HT and without HT). The groupings were compared according to recurrence, clinicopathologic and ultrasound (US) characteristics, and disease free survival.
Results
Of 144 patients, 41 had CLT and 19 had HT. There were 10 patients (6.9%) with tumor recurrence. In both groupings, the presence of calcification was more frequently associated with patients with LT (p=0.041 and 0.047, respectively). In Grouping 2, the mean age at diagnosis was older in patients without HT compared to patients with HT (p=0.032). On multivariate analysis, the presence of LT was not an independent predictor of recurrence in both groupings. For both groupings, pathologic tumor size and taller than wide shape on US were independent predictors of recurrence. The presence of LT in PTC patients did not affect recurrence.
Hashimoto thyroiditis (HT) is a common finding in resected thyroid specimens.1 However, even though other aspects of HT have been extensively studied, there is still controversy on whether patients with papillary thyroid carcinoma (PTC) and concurrent HT are associated with favorable outcomes.2,3,4,5,6,7,8,9,10 Whereas several reports concluded that better prognosis was shown in the patients with the two diseases at the same time,4,6,7,11 the other reports conclude that HT was no significant prognostic factor predicting the outcome of PTC patients.2,3
Autoimmune thyroiditis, of which the pathologic hallmark is lymphocytic infiltration, can show a certain pathologic spectrum from lymphocytic infiltration only (chronic lymphocytic thyroiditis, CLT) to progressive loss of thyroid follicular cells, a concomitant replacement of the gland by lymphocytes, and the formation of germinal centers associated with fibrosis (HT).12 As the disease progresses the cellular infiltrate and the fibroblastic tissue increase, resulting in the disorganization and destruction of thyroid tissue.13 Therefore, this certain spectrum of pathologic features may affect the prognosis of PTC patients in different ways. To the best of our knowledge, no reports have compared CLT and HT with the prognosis of PTC. Therefore, our goal was to investigate the prognosis of PTC patients according to the presence of CLT or HT.
The Institutional Review Board of Severance Hospital approved this retrospective observational study and required neither patient approval nor informed consent for review of patients' images and medical records.
Between April 2003 and December 2004, preoperative ultrasound (US) and pathologic slides were both found available for 356 PTC patients. 119 patients were excluded from this study as they had another coexisting primary site of cancer (n=15), previous surgical history of the thyroid gland (n=14), or follow up loss in 5 years (n=90). For the survival analysis, patients who underwent the same therapy had to be selected for the study. Therefore, among the remaining 237 patients who underwent both total thyroidectomy with radioactive iodine remnant ablation were selected, making a total of 144 patients for the study population.
US images were obtained using 5-12-MHz linear transducers (HDI 5000 and IU-22, respectively; Philips, Bothell, WA, USA). All US examinations were reviewed on a GE Centricity PACS workstation (GE Healthcare, Milwaukee, WI, USA) by one radiologist (K.J.Y.). US features of thyroid cancers were described by composition, echogenicity, margin, calcification, and shape.14 The internal composition was classified as solid and mixed. The echogenicity of a nodule was categorized as iso- or hypoechogenicity (which was defined as iso- or hypoechogenic compared to the normal thyroid gland) and marked hypoechogenicity (which was defined as hypoechogenic compared to the strap muscle). The margin was categorized as well defined and not well defined. Calcification was categorized as presence of calcification and without calcification. The shape was described as wider than tall (which was defined as a ratio of the transverse diameter to the anteroposterior diameter of <1) and taller than wide (which was defined as a ratio of the transverse diameter to the anteroposterior diameter of ≥1).
All surgical specimens of the 144 patients were re-evaluated by one pathologist (K.H.K.). Background thyroid pathology was evaluated based upon the presence of lymphocytic thyroiditis (LT). In this study, LT included both CLT and HT. CLT was defined with the histologic features of the presence of diffuse lymphocytic infiltrate, oxyphilic cells, and formation of lymphoid follicles with germinal centers. HT was defined with the histologic features of diffuse lymphocytic thyroiditis with follicular atrophy, diffuse destruction of thyroid follicles, fibrosis, and follicular cell regeneration.12 Peritumoral inflammatory response was not considered as lymphocytic thyroiditis change.
Patients were followed every six months in the first three years after surgery. Afterwards, follow-up evaluations took place every twelve months. Recurrence was defined with elevated serum Tg, uptake on 131I whole body scan, or cytologic confirmation of PTC. US-guided fine needle aspiration (US-FNA) was performed to obtain cytopathologic specimens to confirm recurrence. Patients with no detectable Tg, no regional or distant metastasis on imaging studies, no evidence of regional recurrence on neck US, and benign cytology results after US-FNA were classified as the no recurrence group.
In this study, LT was divided into CLT and HT for analysis. Two different groupings were formed by two different reference points (CLT and HT) of LT. For Grouping 1, subjects were divided into PTCs without LT (including both CLT and HT) and PTCs with LT. For Grouping 2, subjects were divided into PTCs without HT and PTCs with HT. We used the independent t-test for continuous variables and the chi-square test for categorical variables to compare clinicopathologic characteristics among the two different groupings.
Disease free survivals were calculated by the Kaplan-Meier method and the log-rank sum test according to the presence of LT (including both CLT and HT) or HT. Univariate and multivariate Cox proportional hazard regression models were used to determine the association between clinicopathologic parameters and recurrence during the follow-up period in the two groupings. A two-tailed p value <0.05 was considered statistically significant. Statistical analysis was performed using SAS software (version 9.1.3; SAS Institute, Cary, NC, USA).
Clinicopathologic characteristics of the included 144 patients are summarized in Table 1. The follow-up period ranged from 38 to 92 months (mean, 68.9±8.1 months). Of the 144 patients, 41 had CLT and 19 had HT. There were 10 patients (6.9%) with tumor recurrence. Among the 10 patients with recurrence, one had CLT and two had HT. Table 2 shows the clinicopathologic and US characteristics of the two groupings. CLT or HT was only found in patients of the female gender. In Grouping 2, the mean age at diagnosis was older in patients without HT (p=0.032). In both groupings, the presence of calcification was more frequently associated with patients with LT (including both CLT and HT) or HT (p=0.041 and 0.047, respectively).
The Kaplan-Meier analysis demonstrated that the presence of LT (both CLT and HT) or HT in PTC patients did not alter the probability of recurrence developing (Fig. 1). To identify predictive factors of recurrence, a Cox proportional hazard model was created. Univariate and multivariate analysis results are listed in Table 3 and 4. On multivariate analysis, neither LT nor HT was an independent predictor of recurrence. Pathologic tumor size and taller than wide shape on US were independent predictors of recurrence in both groupings.
Lymphocytic thyroiditis has a certain pathologic spectrum with various degrees of inflammatory change with lymphocytic infiltration.15 Although a number of investigators have classified LT into several types according to different degrees of lymphocytic infiltration, epithelial changes, and fibrosis (Table 5),15,16,17,18,19,20,21 most pathologists have used CLT and HT. CLT is defined when histologic characteristics meet the following conditions; presence of diffuse lymphocytic infiltrate, oxyphilic cells, and the formation of lymphoid follicles or reactive germinal centers.8 HT has been differentiated from CLT and is defined when histologic characteristics meet the following additional conditions along with the characteristics of CLT; presence of Hurthle cells and varying degree of acini atrophy.22
The nature association between LT and PTC is still uncertain. A molecular study concluded that multiple occult tumors exist in HT at high frequency.23 Some studies suggested that chronic thyroiditis will induce malignant transformation by enhancing mitosis and proliferation of thyroid cells.24 Although its nature remains uncertain, studies regarding the relationship between CLT or HT and PTC have been reported. As HT patients showed a higher incidence of thyroid carcinoma, HT was thought to be a predisposing factor in the development of thyroid carcinoma.25 However, there was a report showing that the pathogenesis of PTC itself stimulates lymphocytic infiltration and the autoimmune mechanism was suggested as a result of malignant pathogenesis rather than as a premalignant factor.26 In fact, CLT and HT have been reported to be favorable factors in the prognosis of PTC,4,7,17,27 although the issue remains somewhat controversial.2,3 The presence of CLT or HT in PTC patients has been related to lower T stage, less extrathyroidal extension, or less nodal metastasis compared to patients without CLT.4,7,17,27 Furthermore, disease free survival was found to be significantly longer in PTC patients with coexisting CLT.8 On the other hand, other studies have shown CLT or HT not affecting any prognostic features such as size, angioinvasion, or multifocality, thereby showing no association between the presence of CLT or HT and PTC aggressiveness.2,3 Although many investigators evaluated the relationship between CLT or HT and the prognostic outcomes of PTC patients, they mixed "CLT" with "HT" and in some cases, even categorized them as the same pathologic condition. Also, to our knowledge, there have been no published reports on the relationship between different pathologic grades of LT and the prognostic outcomes of PTC. Therefore, we sought to investigate whether the pathologic grade of LT can affect the outcome of PTC patients along with other clinicopathologic and US features.
Factors influencing a poor outcome of thyroid tumor recurrence include older age, male gender, larger size, cervical node or distant metastasis, extrathyroidal extension, multiplicity of the tumor, and tumor stage.5,28 In our study, larger PTC is significantly associated with a higher recurrence rate of PTC regardless of the presence of CLT or HT, similar to previous reports that show larger PTC nodules having an association with poor prognostic factors.5,29 There have been several reports about the relationship of US characteristics with clinical outcomes. Patients with PTC with benign looking results on US had better prognosis than ones with malignant looking results on US.30 Ill-defined margins or the presence of calcification was associated with lymph node metastasis in PTC patients.31,32 PTCs with taller-than-wide shape on US were associated with extrathyroidal extension and BRAF mutation which may be a poor prognostic factor.33 Our study shows that a PTC patient with taller-than-wide shape on US is more likely to recur with or without LT. Further studies will be needed to verify this.
There are some limitations to our study. First, this is a retrospective study and has selection bias as we excluded patients with other primary malignancies, with previous history of surgery or other procedures at the thyroid gland, or with a follow-up of less than 5 years. Second, all US image analyses were based on the retrospective review of previously taken images. The interpretation of US findings by the reviewer could have been different from the actual US performer. Third, only one pathologist and radiologist reviewed the specimens and US findings. Therefore, interobserver variability should be considered in future studies. Fourth, there was a relatively small number of the study population to compare disease free survival between each group of patients. Further study is needed with larger number of patients in each group to obtain more profound results.
We conclude that there was no relationship between PTC prognosis and different grades of LT. Pathologic tumor size and taller than wide shape on US were independent predictors of PTC recurrence regardless of concurrent LT.
Figures and Tables
Fig. 1
Disease free survivals in patients with or without lymphocytic thyroiditis (LT, including both chronic lymphocytic thyroiditis and Hashimoto thyroiditis) (A) and Hashimoto thyroiditis (HT) (B). Log-rank test p-value of A and B is 0.880 and 0.459, respectively.

Table 1
Clinicopathologic Characteristics in 144 Patients Who Underwent Total Thyroidectomy and with Radioactive Iodine Remnant Ablation
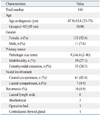
Table 2
Difference in Clinicopathologic and Ultrasound Characteristics between PTC Patients with or without CLT or HT
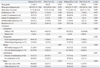
Table 3
Univariate and Multivariate Predictors of Recurrence When LT Including Both CLT and HT Is Considered as One of the Clinicopathologic Parameters
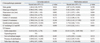
References
2. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126:1070–1076.

3. Del Rio P, Cataldo S, Sommaruga L, Concione L, Arcuri MF, Sianesi M. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008; 33:1–5.
4. Schäffler A, Palitzsch KD, Seiffarth C, Höhne HM, Riedhammer FJ, Hofstädter F, et al. Coexistent thyroiditis is associated with lower tumour stage in thyroid carcinoma. Eur J Clin Invest. 1998; 28:838–844.

5. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.

6. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998; 8:197–202.

7. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999; 84:458–463.

8. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009; 71:581–586.

9. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, et al. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012; 27:883–889.

10. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013; 168:343–349.

11. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995; 80:3421–3424.

12. Rho MH, Kim DW, Hong HP, Park YM, Kwon MJ, Jung SJ, et al. Diagnostic value of antithyroid peroxidase antibody for incidental autoimmune thyroiditis based on histopathologic results. Endocrine. 2012; 42:647–652.

13. Thomson AD. The pathology of Hashimoto's disease. Proc R Soc Med. 1957; 50:950–952.
14. Sohn YM, Yoon JH, Moon HJ, Kim EK, Kwak JY. Mixed echoic thyroid nodules on ultrasound: approach to management. Yonsei Med J. 2012; 53:812–819.

16. Bhatia A, Rajwanshi A, Dash RJ, Mittal BR, Saxena AK. Lymphocytic thyroiditis--is cytological grading significant? A correlation of grades with clinical, biochemical, ultrasonographic and radionuclide parameters. Cytojournal. 2007; 4:10.
17. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001; 25:632–637.
18. Kumar N, Ray C, Jain S. Aspiration cytology of Hashimoto's thyroiditis in an endemic area. Cytopathology. 2002; 13:31–39.

19. Herh SJ, Kim EK, Sung JM, Yoon JH, Moon HJ, Kwak JY. Heterogeneous Echogenicity of the Thyroid Parenchyma Does Not Influence the Detection of Multi-focality in Papillary Thyroid Carcinoma on Preoperative Ultrasound Staging. Ultrasound Med Biol. 2014; 40:884–889.

20. Kim I, Kim EK, Yoon JH, Han KH, Son EJ, Moon HJ, et al. Diagnostic role of conventional ultrasonography and shearwave elastography in asymptomatic patients with diffuse thyroid disease: initial experience with 57 patients. Yonsei Med J. 2014; 55:247–253.

21. Park M, Park SH, Kim EK, Yoon JH, Moon HJ, Lee HS, et al. Heterogeneous echogenicity of the underlying thyroid parenchyma: how does this affect the analysis of a thyroid nodule? BMC Cancer. 2013; 13:550.

22. Kim HS, Choi YJ, Yun JS. Features of papillary thyroid microcarcinoma in the presence and absence of lymphocytic thyroiditis. Endocr Pathol. 2010; 21:149–153.

23. Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. 1997; 107:95–100.

24. Okayasu I, Saegusa M, Fujiwara M, Hara Y, Rose NR. Enhanced cellular proliferative activity and cell death in chronic thyroiditis and thyroid papillary carcinoma. J Cancer Res Clin Oncol. 1995; 121:746–752.

25. Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008; 150:49–52.

26. Tamimi DM. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002; 10:141–146.

27. Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK, Kim MR, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck. 2011; 33:1272–1277.

28. Simpson WJ, McKinney SE, Carruthers JS, Gospodarowicz MK, Sutcliffe SB, Panzarella T. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med. 1987; 83:479–488.
29. Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol. 2011; 18:1916–1923.

30. Nam SY, Shin JH, Han BK, Ko EY, Ko ES, Hahn SY, et al. Preoperative ultrasonographic features of papillary thyroid carcinoma predict biological behavior. J Clin Endocrinol Metab. 2013; 98:1476–1482.

31. Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, et al. Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol. 2009; 16:1348–1355.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download