Abstract
Purpose
Procalcitonin (PCT) is a current, frequently used marker for severe bacterial infection. The aim of this study was to assess the ability of PCT levels to differentiate bacteremic from nonbacteremic patients with fever. We assessed whether PCT level could be used to accurately rule out a diagnosis of bacteremia.
Materials and Methods
Serum samples and blood culture were obtained from patients with fever between August 2008 and April 2009. PCT was analyzed using a VIDAS® B.R.A.H.M.S PCT assay. We reviewed the final diagnosis and patient histories, including clinical presentation and antibiotic treatment.
Results
A total of 300 patients with fevers were enrolled in this study: 58 with bacteremia (positive blood culture) (group I); 137 with local infection (group II); 90 with other diseases (group III); and 15 with fevers of unknown origin (group IV). PCT levels were significantly higher in patients with bacteremia than in those with non-bacteremia (11.9 ± 25.1 and 2.5 ± 14.7 ng/mL, respectively, p < 0.001). The sensitivity and specificity were 74.2% and 70.1%, respectively, at a cut-off value of 0.5 ng/mL. A serum PCT level of < 0.4 ng/mL accurately rules out diagnosis of bacteremia.
The prompt diagnosis and treatment with an appropriate antimicrobial agent is important in reducing morbidity and mortality associated with bacteremia.1 Microbiologic culturing requires at least 24-48 hours, and negative cultures do not exclude the presence of infection.2 Moreover, only 5-10% of blood cultures performed in hospitals show microorganisms.3 Thus, a rapid and reliable test to rule out bacteremia would be helpful in decision making. Procalcitonin (PCT), the precursor of the hormone calcitonin, is produced by C-cells of the thyroid gland or neuroendocrine cells in the lung or intestine.4,5 Although only very few PCT molecules are released into circulation in a normal state, serum PCT concentrations increase in patients with bacterial and viral infections; systemic bacterial infection, especially, can induce higher concentrations of serum PCT.6 PCT concentration has been reported to be useful for the early diagnosis of bacteremia and decisive initial antimicrobial therapy.7-11 Furthermore, following administration of antimicrobial agents with serial measurements of PCT levels can help to protect against emerging antimicrobial-resistant strains by restricting unnecessary antibiotic use.12 A new automated rapid quantitative assay, VIDAS® B.R.A.H.M.S PCT assay (bioMérieux, Marcy L'Etoile, France), is based on an enzyme-linked fluorescent immunoassay (ELFA) and has a functional sensitivity of 0.09 ng/mL.
The aim of this study was to assess the ability of PCT level to differentiate bacteremia from non-bacteremia in patients with fever using this new assay. We also assessed whether PCT level could be used to rule out the diagnosis of bacteremia.
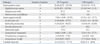
This study was carried out from August 2008 to April 2009 at a tertiary hospital in Korea. All 300 patients with acute fever (temperature ≥ 38℃) were enrolled in this study, and blood cultures were performed for all of them. These patients were not administered antimicrobial agents prior to blood culture. Their medical records were reviewed for clinical diagnosis, laboratory findings, and antimicrobial treatments. The patients were classified as bacteremia (group I), localized infection (group II), other disorders (group III), and fever of unknown origin (FUO, group IV) according to the final diagnosis.
Blood samples were obtained from each patient to determine serum PCT level and C-reactive protein (CRP) level at the same time that the samples were obtained for culture. PCT level was measured via an automatic analyzer, the VIDAS® B.R.A.H.M.S PCT assay (bioMérieux, Marcy L'Etoile, France). The lower limit of detection of the assay was 0.05 ng/mL. CRP level was measured using CRPα Auto (Wako Pure Chemical Industries, Ltd., Osaka, Japan) by TBA-200 FR NEO (Toshiba Medical Systems Corporation, Tochigi-ken, Japan). Blood cultures were processed in more than two pairs of bottles and were incubated in aerobic and anaerobic conditions in an automatic analyzer, BACTEC 9240 Blood Culture System (Becton Dickinson Diagnostic Instrument Systems, Sparks, MD, USA). Blood samples were secured in Bactec culture vial types Plus Aerobic/F (enriched soybean-casein digest broth), Bactec Plus 277Anaerobic/F (prereduced enriched soybean-casein digest broth) or Peds Plus F (enriched soybean-casein digest broth), and bacterial growth was monitored. From the positive samples a small volume was inoculated onto blood agar and MacConkey agar plates and incubated overnight at 37℃. MicroScan WalkAway-96 System (Dade Behring, Sacramento, CA, USA) was used to identify the bacteria species. If a single blood culture yielded coagulase-negative staphylococci (CNS), viridans streptococci, Bacillus spp., Corynebacterium spp. or Propionibacterium spp., the culture was considered to have been contaminated.
The comparison of group differences for continuous variables was performed using one-way ANOVA. Receiver operating characteristic (ROC) curves were plotted for PCT and CRP, and diagnostic accuracy was assessed by calculating the areas under the ROC curves (AUCs). Because we wanted to evaluate the capacity of PCT to rule out the diagnosis of bacteremia, we sorted the values of PCT into ten deciles and calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) associated with the nine cut-off values separating the ten deciles. All statistical analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
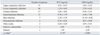
Of 300 patients enrolled in the study, 23 were excluded because they were diagnosed as having disorders that were not suspected to be infection: trauma, hemorrhage, burns, or immunologic disease. Twenty-five positive blood cultures were excluded because they were considered to have been contaminated; 23 coagulase negative streptococci in single blood culture and 2 Bacillus spp.. None of the remaining 252 patients were enrolled more than once, and all were classified as either bacteremia (group I, n = 31), localized infection (group II, n = 129), other disorders (group III, n = 83), or FUO (group IV, n = 9)(Table 1). Thirty-one patients in the bacteremic group had 16 Gram-negative bacilli (Escherichia coli in nine cases; Klebsiella pneumoniae in five; Serratia marcescens in one; Salmonella Typhi in one), seven glucose non-fermenting gram-negative bacilli (Acinetobacter baumannii in three cases; Pseudomonas aeruginosa in two, Acinetobacter lwoffii in two), six gram-positive cocci (Staphylococcus aureus in four cases; Streptococcus mitis in one; Enterococcus casseliflavus in one), and two cases of Candida tropicalis (Table 2). The PCT levels in gram-positive and gram-negative bacteremia were 26.26 ng/mL (0.22-125.38 ng/mL) and 7.95 ng/mL (< 0.05-47.97 ng/mL), respectively, with the levels significantly higher in gram-positive (p = 0.009). However, CRP levels were not significantly different between two types of bacteremia (p = 0.659).
The mean serum PCT level was 11.9 ± 25.1 ng/mL in the bacteremic group (group I), which was significantly higher, than in the non-bacteremic groups (groups II, III, and IV), which was 2.5 ± 14.7 ng/mL (p < 0.001). The most significant difference was found between PCT levels in the bacteremic group (group I) and in the localized infection group (group II), which was 1.2 ± 3.5 ng/mL (p < 0.001). There were no significant differences between the bacteremic group and the non-bacteremic groups (group II, III, and IV) with respect to CRP levels (p = 0.298).
The PCT levels between the sites of localized infections were not significantly different, but the CRP levels showed significant differences (Table 3). The infection sites of patients with localized infection were the upper respiratory tract in 31 cases; the lower respiratory tract in 46 cases; the urinary tract in 27 cases; the gastrointestinal tract in 11 cases; the skin infectionin 7 cases; osteoarticular infection in 3 cases; otitis media in two cases; infective endocarditis in one case; and malaria in one case.
Fig. 1 presents the ROC curves of the PCT and CRP concentrations for discrimination between patients in the bacteremic group (group I) and patients in the non-bacteremic groups (groups II, III, and IV). The AUCs were 0.753 and 0.696, respectively. The AUCs for discrimination of bacteremia from localized infection were 0.761 and 0.739, respectively (Fig. 1).
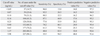
Sensitivity, specificity, PPV and NPV of PCT levels for the nine cut-off values are shown in Table 4. The highest NPV was 95.4%, which was associated with a cut-off value of 0.4 ng/mL. Sensitivity, specificity, PPV and NPV at the cut-off value 0.5 ng/mL, which was recommended by the manufacturer, were 74.2%, 70.1%, 26.1% and 95.1%, respectively.
Early diagnosis of bacteremia in patients with fever is important for reducing the morbidity and mortality associated with bacteremia and its complications.1 The gold standard for diagnosis of bacteremia is isolation and identification of organisms; however, blood cultures lack sensitivity, are time consuming, and can be problematic due to skin microorganism contamination.13 The diagnosis of bacteremia in patients presenting with fever has been reliant on a combination of clinical examination and laboratory parameters, such as CRP level and erythrocyte sedimentation rate (ESR). However, these parameters lack accuracy for early diagnosis of bacteremia.14 PCT has recently come to interest as a possible marker of the systemic inflammatory response to infection.7-11 Although many studies have established that PCT level can be used to identify bacterial infections in patients with sepsis,7,8,11,15 only a few studies have evaluated the capacity of PCT findings to rule out bacteremia in outpatients with fever. A previous study evaluated PCT level as an early predictive marker of bacteremia, PCT had NPVs of 99% and 96% when the PCT cut-off values were 0.2 ng/mL and 0.5 ng/mL, respectively.16 Another study evaluated the performance indices of PCT in a prospective analysis of 300 hospitalized medical patients with fever.17 The sensitivity, NPV, and AUC of PCT for the diagnosis of bacteremia were 75%, 90%, and 0.7, respectively, when using 0.5 ng/mL as the cut-off value. Similarly, we found in this study that the sensitivity, specificity, PPV, and NPV were 74.2%, 70.1%, 26.1% and 95.1%, respectively. Although PCT levels were shown to have a higher tendency, we believe that its power to discriminate bacteremia from localized infection was sufficient. The ROC curve for differentiating patients with bacteremia from patients with localized infection based on the PCT level had an AUC of 0.769.
The present study showed that PCT levels in gram-positive bacteremia could be significantly higher than those in gram-negative bacteremia, which is contradictory to the results of a previous study.18 However, the number of the patients with gram-positive bacteremia was too small. It is therefore difficult to generalize for all patients with bacteremia based on our results.
In a previous study, PCT levels were determined using an immunoluminometric assay (LUMItest, BRAHMS, Henningsdorf, Germany), and the functional sensitivity of this method was 0.5 ng/mL. However, because 0.5 ng/mL exceeds the average normal value by more than ten-fold, many mild changes in PCT values are missed.19 More sensitive detection methods are needed for the accurate assessment of the clinical usefulness of PCT values.20 We evaluated the diagnostic usefulness of PCT for bacteremia using a developed method, the enzyme-linked fluorescent assay (ELFA). The functional sensitivity of ELFA is 0.09 ng/mL, which is close to the upper normal value.
Although lacking diagnostic specificity, CRP is known as a sensitive marker of infection or inflammation.21 In this study, there was no significant difference between mean CRP concentrations of patients with bacteremia and non-bacteremia. ROC analysis confirmed that the diagnostic utility of CRP was inferior to that of PCT in discriminating between these conditions.
We found that a PCT value of < 0.4 ng/mL could rule out bacteremia with a high degree of accuracy, because the NPV associated with this threshold was as high as 95.4%. Similarly, a previous study reported that < 0.4 ng/mL was the best cut-off value for ruling out bacteremia.12 Determination of the PCT level seems to be a consistent tool for ruling out bacteremia in patients with acute fever.
In conclusion, PCT level could be a reliable marker to rule out or predict bacteremia in patients presenting with acute fever, and therefore, help in deciding the appropriate use of antimicrobial agents. More prospective and large-scale studies are needed to better define the usefulness of PCT levels in various clinical settings.
Figures and Tables
Fig. 1
(A) Receiver operator characteristic curve demonstrating sensitivity as a function of one-specificity for discriminating patients with blood culture positivity based on procalcitonin (PCT) and C-reactive protein (CRP) levels. PCT and CRP had areas under the receiver operator characteristic curve of 0.753 and 0.696, respectively. (B) Receiver operator characteristic curve demonstrating sensitivity as a function of one-specificity for discriminating patients with bacteremia from patients with localized infection based on PCT and CRP. PCT and CRP had areas under the receiver operator characteristic curve of 0.769 and 0.746, respectively.

References
2. Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005. 351:17–29.

3. Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997. 10:444–465.

4. Jacobs JW, Lund PK, Potts JT Jr, Bell NH, Habener JF. Procalcitonin is a glycoprotein. J Biol Chem. 1981. 256:2803–2807.

5. Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000. 26:1193–1200.

6. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993. 341:515–518.

7. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000. 26:Suppl 2. S148–S152.

8. Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, et al. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008. 14:244–249.

9. Harris KR, Digard NJ, Lee HA. Serum C-reactive protein. A useful and economical marker of immune activation in renal transplantation. Transplantation. 1996. 61:1593–1600.
10. Jongwutiwes U, Suitharak K, Tiengrim S, Thamlikitkul V. Serum procalcitonin in diagnosis of bacteremia. J Med Assoc Thai. 2009. 92:Suppl 2. S79–S87.
11. Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003. 31:1737–1741.

12. Chirouze C, Schuhmacher H, Rabaud C, Gil H, Khayat N, Estavoyer JM, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis. 2002. 35:156–161.

13. Rowther FB, Rodrigues CS, Deshmukh MS, Kapadia FN, Hegde A, Mehta AP, et al. Prospective comparison of eubacterial PCR and measurement of procalcitonin levels with blood culture for diagnosing septicemia in intensive care unit patients. J Clin Microbiol. 2009. 47:2964–2969.

14. Bates DW, Sands K, Miller E, Lanken PN, Hibberd PL, Graman PS, et al. Academic Medical Center Consortium Sepsis Project Working Group. Predicting bacteremia in patients with sepsis syndrome. J Infect Dis. 1997. 176:1538–1551.

15. Müller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000. 28:977–983.

16. Liaudat S, Dayer E, Praz G, Bille J, Troillet N. Usefulness of procalcitonin serum level for the diagnosis of bacteremia. Eur J Clin Microbiol Infect Dis. 2001. 20:524–527.

17. Bossink AW, Groeneveld AB, Thijs LG. Prediction of microbial infection and mortality in medical patients with fever: plasma procalcitonin, neutrophilic elastase-alpha1-antitrypsin, and lactoferrin compared with clinical variables. Clin Infect Dis. 1999. 29:398–407.

18. Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008. 8:38.

19. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008. 36:941–952.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download