Abstract
Inflammation is known to participate in the mediation of a growing number of acute and chronic neurological disorders. Even so, the involvement of inflammation in the pathogenesis of epilepsy and seizure-induced brain damage has only recently been appreciated. Inflammatory processes, including activation of microglia and astrocytes and production of proinflammatory cytokines and related molecules, have been described in human epilepsy patients as well as in experimental models of epilepsy. For many decades, a functional role for brain inflammation has been implied by the effective use of anti-inflammatory treatments, such as steroids, in treating intractable pediatric epilepsy of diverse causes. Conversely, common pediatric infectious or autoimmune diseases are often accompanied by seizures during the course of illness. In addition, genetic susceptibility to inflammation correlated with an increased risk of epilepsy. Mounting evidence thus supports the hypothesis that inflammation may contribute to epileptogenesis and cause neuronal injury in epilepsy. We provide an overview of the current knowledge that implicates brain inflammation as a common predisposing factor in epilepsy, particularly childhood epilepsy.
Epilepsy is a neurologic condition of diverse etiologies that affects about 1% of the global population.1 Approximately 325,000 children from 5 to 14 years of age have active epilepsy, and the majority of adults with epilepsy experience childhood onset of seizures.2 The median age of seizure onset is between 5 and 6 years.3 A single unprovoked seizure during childhood is common, occurring in 4 - 10% of children.4 Children in their first year of life, particularly, are at the highest risk for developing epilepsy.
Although the central nervous system (CNS) used to be considered an immunoprivileged system due to the presence of the blood-brain barrier (BBB), graft acceptance, a lack of conventional lymphatic drainage, and relatively low levels of monocytes and lymphocytes, it is becoming clear that immune and inflammatory reactions do occur in the CNS, either intrinsically from the brain itself or acquired from systemic circulation through a damaged BBB. While the role of inflammation in the pathophysiology of human epilepsy remains hypothetical, inflammatory and immune reactions in the brains do occur in human epilepsy patients and in experimental models of epilepsy.
Acute inflammatory reaction after seizures has long been suspected due to clinical observation of pleocytosis, without any evidence of infection, in the cerebrospinal fluid (CSF) and peripheral blood of patients with recent generalized convulsions. Steroids or adrenocorticotropic hormone (ACTH), with a suppressive effect on inflammation or immune reactions, have been used to effectively treat children with intractable epilepsy. Various common pediatric infectious or autoimmune diseases are often heralded by seizures at the onset or accompanied by seizures during the course of the illness.
The BBB consists of morphologically noninfestrated endothelial cells with interendothelial tight junctions, and its maintenance depends on normal functioning of pericytes, perivascular microglia, astrocytes, and the basal lamina. Under normal conditions, the BBB protects the CNS by regulating the entry of plasma-born substances and immune cells.5 Astrocytes are thought to act as important regulators of the balance between endothelial stability and permeability of the BBB. A high transendothelial barrier can be reintroduced in human or bovine endothelial cell monolayers cultured in astrocyte-conditioned media, suggesting that astrocyte-derived soluble factors may contribute BBB characteristics to endothelial cells.6 Transient changes have been demonstrated in the physiology and structures of the BBB in various CNS injuries such as status epilepticus, infections, and traumatic and ischemic events.5 An impaired BBB and inflammatory state are common features of neurological diseases associated with the late onset of epilepsy.7 Proinflammatory cytokines are elevated in experimental animal brains after ischemia8 and in the CSF from stroke9 and epilepsy10 patients. Cytokine release causes subsequent up-regulation of endothelial and neutrophil adhesion molecules in human cerebrovascular endothelial cells during hypoxic injury,11 leading to transmigration of leukocytes across the endothelium and the BBB. Leukocyte recruitment may trigger signal transduction cascades, resulting in tight junction disorganization and BBB breakdown. Although the mechanism of delayed onset of epilepsy remains unclear, available data suggest that inflammation and breakdown of the BBB are necessary components of epileptogenesis following brain injury. Further work is needed to determine whether BBB breakdown is a pre-requisite for future development of epilepsy and to elucidate the potential for prophylactic treatment during the latent period following an injury to prevent epilepsy.
The incidence of arterial ischemic stroke and cerebral sinovenous thrombosis has increased to 2 to 6 per 100,000 children a year during the past 10 years.12 Even in neonates, stroke affects as many as 1 in every 4000 live births.13 Common risk factors include congenital heart disease and sickle cell disease. Stroke in infancy and childhood adversely affects development; neurological deficits occur in 60% of children and 10 - 25% will experience recurrent stroke.14 Treatment is empirically provided with antithrombotic drugs.
In perinatal stroke, neonatal seizures are the most common clinical symptom. Neonatal seizures in a setting of arterial stroke are mostly focal and may occur in the absence of other signs of neonatal encephalopathy. Of infants with ischemic cerebral infarcts proven in autopsy studies, 25 - 40% are diagnosed with neonatal seizures.15 Some infants with a normal neurological examination in the neonatal period may be diagnosed in later months with asymmetry of reach and grasp, failure to reach developmental milestones, or post-neonatal seizures.16 Neonatal seizures and abnormal neurologic examination at discharge are two risk factors for later neuro-developmental disabilities and chronic epilepsy.17 Childhood epilepsy is a frequent resulting morbidity in perinatal stroke. In one cohort study of 64 perinatal stroke patients, 75% of children presented with neonatal seizures and 67% developed epilepsy after 6 months of age.18 The median age at onset of delayed epilepsy was 16 months.
In childhood stroke, seizures commonly occur acutely and may be the presenting symptom of stroke. A study of 73 children 17 years old and younger with acute hemiplegia from stroke found that 50% of patients had at least one seizure and 29% had recurrent seizures.19 Time to onset of delayed seizures and development of epilepsy ranged from 4 months to more than 10 years in 42 children after unilateral hemispheric stroke.20
Proinflammatory cytokines IL-1β and IL-6 increase in CSF from stroke patients within the first 24 h after the beginning of symptoms.21 Systemic injections of progesterone, a neurosteroid, improve cognitive recovery after stroke and decrease molecular indicators of neuronal damage in rats,22 suggesting immunosuppression as a possible treatment strategy to prevent late sequelae.
SLE is the most common rheumatic disease in children.23 The major organ systems involved in childhood SLE are similar to adult SLE, but the frequency of multiple organ involvement and severity of the disease are greater in children than adults.23 General clinical features include broad variations of rash, arthritis, constitutional symptoms, renal disease, and cardiovascular, pulmonary, and neuropsychiatric involvement. The prevalence of epilepsy in SLE patients is 10 - 20%, 8 times higher than in the general population. Notably, seizures can precede the diagnosis of SLE, and 5 - 10% of patients experience seizures several years before the clinical onset of SLE.24 This finding suggests that long-term treatment with antiepileptic drugs may have precipitated SLE, or alternatively, that epilepsy and SLE are both manifestations of a genetically determined predisposition to altered immunity and inflammatory reaction. Epilepsy in SLE is significantly associated with anti-phospholipid antibodies, and the presence of anti-phospholipid antibodies highly correlates with abnormal MRI findings of the brain.24 Patients with anti-phospholipid antibodies are at risk of thromboembolic manifestations, intrauterine fetal loss, and thrombocytopenia, a combination termed anti-phospholipid syndrome. In addition, anti-cardiolipin antibodies are found in 30 - 60% of SLE patients, and epilepsy is 3 times more frequent in patients with anti-cardiolipin antibodies than those without.25 These findings raise the possibility that autoantibodies may trigger seizures and contribute to epileptogenesis.
Hashimoto thyroiditis is the most common thyroiditis in children and adults, affecting 1.2% of school-aged children.26 Hashimoto encephalopathy is a syndrome associated with high antithyroid antibody titer that can either begin abruptly, in the form of seizures or agitation, or develop gradually, in a relapsing-remitting manner manifested as cognitive deterioration and psychiatric illness. The occurrence of Hashimoto encephalopathy is unrelated to the patient's thyroid function status, and most patients respond dramatically to corticosteroid therapy.26 The therapeutic efficacy of immunomodulation suggests a causative role of activated immunity and autoantibodies for the neurological symptoms in Hashimoto thyroiditis, including seizures and epilepsy.
Behçet's disease is a chronic systemic inflammatory disorder of unknown etiology defined by the classical triad of recurrent oral aphthous, genital ulcers, and inflammatory eye disease. The prevalence of CNS disease, often associated with significant morbidity, varies between 2.9% and 44%, with male predominance.27 In 22 patients with neuro-Behçet's disease, 27% suffered either single or recurrent seizures.28 CSF pleocytosis occurred in 50% of patients, and most showed improvement after immunosuppressant therapy.
Rasmussen's encephalitis is a prototype of inflammatory epilepsy. The autoimmune nature of this condition was suspected after the discovery of autoantibodies against glutamate receptor, GluR3, one of the AMPA (α-3-hydroxy-5-methyl-4-isoxazolepropionic acid) subunits. Subsequently, anti-GluR3 antibodies have been detected in two other epilepsy syndromes, early-onset noninflammatory focal epilepsy and catastrophic infantile epilepsy.29 Anti-cardiolipin antibodies, found in SLE patients, are also found in epilepsy patients, both adults30 and children.31,32 Furthermore, increased levels of anti-nuclear antibodies have been reported in epilepsy patients.30 Anti-B2-glycoprotein I antibodies, specific for thrombosis-mediated events, have been demonstrated in SLE patients with epilepsy33 as well as other epilepsy patients.30 Long-term immunotherapy such as intravenous γ-globulin and corticosteroids may be beneficial for autoantibody-positive epilepsy patients.
Febrile seizures are the most common cause of seizures in children, affecting 2 to 5% of children.34 The threshold to febrile seizures is dependent on the height of the body temperature, but the threshold varies with individuals and according to age and maturation.35 A genetic susceptibility to inflammation may influence the threshold convulsive temperature. Seventeen to 30% of febrile seizure patients have a family history of febrile seizures.35 A biallelic polymorphism in the promoter region of IL-1β at the -511 position that can increase IL-1β production occurs more frequently in patients with prolonged febrile convulsions.36,37 In experimental animals, intraventricular injection of IL-1β reduces the seizure threshold in 14-day old mice subjected to hyperthermia, while IL-1receptor knock-out mice have higher seizure thresholds, supporting the role of proinflammatory cytokines in triggering febrile seizures.38
Viruses as being increasingly implicated as causative agents of febrile seizures. Neurotropic viruses, such as the herpesviruses and influenza A, are commonly associated with febrile seizures in the United States and Asia.39,40 Fever induced by viral infection is regulated by components of the immune response, particularly proinflammatory cytokines. Proinflammatory cytokines are higher in influenza-associated febrile seizures, further suggesting a causative role for cytokines in the pathogenesis of febrile seizures. Common pathogens and causes associated with febrile seizures are detailed below.
HHV-6 causes exanthema subitum, an acute febrile illness affecting more than 90% of children before 2 years of age, the period of greatest susceptibility to febrile seizures. The incidence of convulsions among exanthema subitum varies from 2 to 50%.35,39,41,42 The incidence of primary HHV-6 infection is similar between first simple febrile seizure patients and age-matched controls.43 HHV-6 DNA, however, is detected more frequently in CSF of patients with three or more seizures than those with a single febrile seizure.44 This finding suggests that HHV-6 may invade the brain during the acute viremic phase of exanthema subitum and then reactivate when triggered and provoke recurrent febrile seizures.
Influenza virus A is a frequent cause of febrile seizures in Japan45 and China.40 During 1997-1998, influenza A accounted for 35 - 44% of hospital admissions for febrile seizures in Hong Kong.40 Influenza-associated febrile seizures are often prolonged and complex, independent of the severity of the viral infection.45 IFN-α and IL-6 are significantly higher in patients with febrile seizures compared to those without.46 This finding suggests that influenza-associated febrile seizures may be the result of systemic immune responses, and that IFN-α and IL-6 are involved in the pathogenesis of febrile seizures (in influenza, at least).
Vaccines, including measles-mumps-rubella (MMR) and diphtheria-tetanus-pertussis (DTP), are associated with an increased risk of febrile seizures in the first three years of life.47 MMR-associated seizures often occur at 7 to 14 days after vaccination, while DTP-associated seizures typically occur on the day of vaccination.48 Children experiencing febrile seizures associated with vaccination have greater rates of family history of febrile seizures and a high risk for recurrent febrile seizures47,49 Although it remains unclear whether vaccination itself represents a risk, or if fever after vaccination is necessary for seizures, immunization-provoked seizures may imply a role for systemic inflammation and genetic susceptibility in the induction of febrile seizures after vaccination.
Benign seizures associated with diarrheal illnesses are afebrile generalized tonic-clonic convulsions that occur between the 1st and the 5th sick day of viral gastroenteritis in previously healthy young children 6 months to 3 years of age.50 Rotavirus antigen has been detected in the majority of patients with this condition and rotaviral RNA has been found in patients' CSF.51 Rotavirus enterocolitis is the most common enterocolitis among young children under 2 years of age. Rotavirus has tropism for astrocytes in astrocytoma cell lines,52 and inoculation of rotavirus strain 2 into the brains of live monkeys can induce transient symptoms reminiscent of typical encephalitis (typical infection of the CNS).53 Since only 6.4% of children with rotavirus gastroenteritis present with afebrile benign seizures,54 it may be that only a few specific strains of rotavirus can infect both the CNS and gastrointestinal system or that host susceptibility factors make a subset of children more prone either to CNS invasion by rotavirus or to specific immune responses to rotavirus.
Nearly 30% of epilepsy patients are refractory to conventional anti-epileptic drugs, and many alternative treatments have been tried to control epilepsy.55 Immunotherapy, such as corticosteroids and ACTH, has been used to treat epilepsy since ACTH was first reported to have beneficial effects in the treatment of infantile spasms in 1950.56 The mechanism behind the anticonvulsant action of corticosteroids or ACTH remains elusive. Possibilities include (1) stimulation of glucocorticoid synthesis that interacts with CNS steroid receptors, which then influences voltage-dependent calcium channels; (2) stimulation of neurosteroid synthesis in glia and neurons that modulate GABAA receptors; (3) down-regulation of corticotrophin releasing hormone (CRH) that has proconvulsant activity in the immature brain; or (4) immunomodulation.57-60
Rasmussen's encephalitis is a severe, progressive focal epilepsy of unknown origin that leads to asymmetric cortical atrophy and deterioration of motor and cognitive function.61 The current histopathological criteria of Rasmussen's encephalitis include the presence of T-cell-dominated inflammation, microglial activation and formation of microglial nodules, neuronal loss, and astrocytic activation. Recently, astrocytic apoptosis and subsequent loss have been demonstrated as a specific feature of Rasmussen's encephalitis.62 A specific attack by cytotoxic T lymphocytes may be one possible mechanism responsible for astrocytic degeneration in Rasmussen's encephalitis.62
Antibodies to GluR3 have been found in the serum of some Rasmussen's encephalitis patients, and repeated plasma exchanges can reduce serum titers of GluR3 antibodies, decrease seizure frequency, and improve neurologic function.63 Immunization of animals with GluR3 induces a disorder resembling the human disease.63 At the same time, a recent study reported that anti-GluR3 antibodies are not specific for Rasmussen's encephalitis. High antibody titers characterize a subgroup of non-Rasmussen's encephalitis patients with "catastrophic" epilepsy and appear to be a marker for intractable epilepsy not limited to Rasmussen's encephalitis. Anti-GluR3B peptide antibodies are significantly associated with seizure frequency.29
There is no effective medical treatment for Rasmussen's encephalitis, except perhaps steroids, which can be useful when given early in the course of the disease.64 A long term follow-up of 11 Rasmussen's encephalitis patients who received steroids showed that 45% of patients had significant improvement of motor function and reduction of seizure frequency with disappearance of epilepsia partialis continua, while 55% patients had no benefit from steroid therapy and ultimately underwent hemispherotomy. Two initial responders to steroid treatment experienced progressive recurrence of seizures one to four years after the discontinuation of steroids and received a hemispherotomy.64
Infantile spasms are a unique, age-specific epilepsy of early infancy. The electroencephalogram (EEG) characteristically shows hypsarrhythmia-disorganized, chaotic, high voltage polymorphic delta and theta rhythms with superimposed multifocal spikes and wave discharges. The onset of spasms is frequently associated with neuro-developmental regression. The incidence varies from 0.25 to 0.60 per 1,000 live births,65 and the prevalence rate is 0.15 to 0.2 per 1,000 children age 10 or younger.66 Infantile spasms develop in infants with a variety of CNS pathologies, including structural abnormalities, prenatal and postnatal infection, stroke, trauma, and chromosomal anomalies.67 This disease entity of uniform semiology with diverse causes suggests that infantile spasms are an age-specific but cause-nonspecific final common response of the brain to insults.68,69
ACTH is a well-known effective treatment for infantile spasms that not only results in seizure control, but also improves both behavior and background EEG.70 Meta-analysis reveals that ACTH is probably effective for short-term treatment of infantile spasms and leads to resolution of hypsarrhythmia.71 Time to response is usually two weeks. Oral steroids can render 30 to 40% of patients seizure-free.72,73 Further, early use of steroids is more effective; patients treated within one month of spasm onset had a better outcome than those treated after more than one month.74 A study of 18 children with Down's syndrome and infantile spasms showed that spasm control was easier, subsequent seizures were less persistent, developmental quotients were higher, and a score of autistic features was lower in children treated early.75
Lennox-Gastaut syndrome (LGS) is diagnosed in children who have a minimum of two seizure types (usually atonic or astatic (drop) seizures, nocturnal tonic seizures, and atypical absence seizures), mental retardation, and bursts of generalized slow spike-waves on EEG.76 LGS is one of the epilepsy syndromes most resistant to the available antiepileptic medications. Corticosteroids and ACTH may be effective in treating children with LGS.55 When 10 children with LGS and intractable seizures were treated with prednisolone for 12 weeks, 7 achieved seizure freedom and the other 3 experienced seizure reduction.77 The patients' clinical improvement was generally reflected in an improvement in their EEGs. Another study of 45 children with LGS who received ACTH for 2 to 8 weeks found that while 51% of patients became seizure free for over 10 days, 78% of these children later relapsed.78
Landau-Kleffner syndrome is an acquired epileptic aphasia presenting as progressive loss of speech in a previously well child with an abnormal and usually continuously epileptic EEGs during slow wave sleep with or without apparent seizures.79 There are reports that corticosteroids improved both clinical features of the syndrome as well as the EEG, especially when instituted early.80,81
Continuous spike-waves during slow sleep syndrome (CSWSS) is a rare, sporadic childhood epilepsy characterized by the presence of spike-waves during at least 85% of slow sleep and, clinically, by the existence of neuropsychological and behavioral disorders.82 LKS and CSWSS share some common features, including first appearance in childhood and mild epilepsy associated with severe neuropsychological disturbances. There are several case reports that CSWSS also responds to treatment with corticosteroids or ACTH, not only in terms of seizures and EEG, but also in language skills.83
Myoclonic or myoclonic-astatic seizures have also been treated with corticosteroids, possibly because of the frequent daily seizures, acute encephalopathic presentation, medical intractability, and tendency of the seizures to result in physical injury. In a study of myoclonic epilepsy, 34 children out of 64 were treated with prednisone and the remaining 30 children with ACTH.84 Seizures were effectively controlled in 73% of children treated with ACTH, but in none treated with prednisone.84 In another study of nine patients with myoclonic epilepsy treated with steroids, five became seizure free, two showed a reduction, and the remaining two exhibited no change.77 In contrast, only a transient improvement was reported in 5 of 84 patients with myoclonic absence epilepsy treated with ACTH.85 In a study of children with absence epilepsy, seven were treated with steroids. All improved and five became seizure free.77 In 32 children with intractable epilepsy (infantile spasms excluded) treated with ACTH, 25% of patients became seizure-free and 47% showed significant reductions of seizures.86
In many animal models of epilepsy, acute seizures cause glial activation and increased expression of transcription factors and cytokines that coordinate inflammatory responses.87,88 After status epilepticus, members of the Toll-like receptor (TLR) family are significantly up-regulated in the microglia, leading to transcriptional activation of cytokines, chemokines, MHC class I and II, and costimulatory molecules.89 The activated glia and elevated cytokines, in turn, contribute to seizure-related hippocampal pathology, such as neuronal death, neuronal birth, reactive gliosis, and mossy fiber sprouting.90-93 Accumulating experimental data also suggest that seizure-induced glial activation and up-regulation of pro-inflammatory cytokines can lead to neuronal excitability and neuronal injury either directly, by interacting with glutamatergic neurotransmission, or indirectly, by activating gene transcription.
Microglia are myeloid lineage cells that comprise approximately 12% of the brain. Resting microglia with a ramified morphology are responsible for immune surveillance94 and are activated at a very early stage in response to injury or immunological stimuli with transformation to an amoeboid shape.95 Activated microglia up-regulate the expression of surface molecules, such as complement receptors and major histocompatibility complex (MHC) molecules, and release a variety of pro-inflammatory and cytotoxic soluble factors.96 MHC class-I and -II, IL-1, IL-2, IL-6, TGF-β1, the complement components and their receptors, M-CSF, and GM-CSF are all molecules considered to be signals in the activation process.95
Widespread microglial activation accompanied by neuronal injury occurs after acute seizures.88,97,98 As a rapid response, activation of microglia may be responsible for neurodegeneration rather than a consequence of neurodegeneration. Within four hours after KA-induced status epilepticus, glial activation and cytokine expression are found in the hippocampus (Fig. 1).99 Neuronal injury is detected 12 - 24 hours following status, many hours after cytokines are induced in the glia.99 Status epilepticus, prolonged seizures over 30 minutes, can cause neuronal death100 through glutamate-mediated excitotoxicity, necrosis, and activation of apoptosis.101 One to three days after status epilepticus, both neuronal and astrocytic death are observed in the dentate hilus within the hippocampus.102,103 Injured neurons and glia and their fragmented DNA are rapidly cleared by activated microglia.93 The impaired neurogenesis in inflammation can be effectively restored by systemic administration of the tetracycline derivative minocycline, a specific inhibitor of microglia activation.104 These findings suggest that microglial activation associated with inflammation induces neuronal injury and suppresses neurogenesis. The occurrence of spontaneous seizures has been correlated with the extent of glial activation, as well as astrocyte and neuron degeneration in the hippocampus, and blockade of neuronal death failed to prevent epileptogenesis.105,106
Cytokines released by microglia influence astrocytic function and proliferation. Increased levels of IFN-γ, IL-1, IL-2, IL-6, TNF-α, and M-CSF are associated with astrogliosis. IL-1Ra, an IL-1 receptor antagonist, is sufficient to prevent astroglial proliferation, suggesting a pivotal role of IL-1 in astrocyte activation.107 In addition, these cytokines may modulate glutamate homeostasis by regulating glutamate receptors and transporters in astrocytes.108,109 Impaired handling of extracellular glutamate by gliotic astrocytes may lead to neuroexcitability and excitotoxic neuronal damages resulting from excessive glutamate levels.110 These findings suggest that activation of microglia and the resultant increase in cytokines may influence epileptogenesis by altering glutamatergic transmission indirectly through modulating astrocytes.
For over 100 years, astroglial proliferation has been observed as a pathognomonic finding in surgically resected hippocampi in patients with intractable mesial temporal lobe epilepsy. Once regarded as an inert scar and consequence of the healing process after neuronal degeneration,111 recent studies112,113 suggest that reactive astrogliosis and modified astroglial function may have an important role in the generation and spread of seizure activity.
Astrocytes are a critical component of the BBB and have many important roles in glutamate and potassium uptake and the production of growth factors, cytokines, and extracellular matrix proteins.104 In response to immunologic challenges or brain injuries, astrocytes proliferate and become hypertrophic and fibrillary. Direct stimulation of astrocytes by photolysis of caged calcium and glutamate release in acute seizure models can induce a paroxysmal depolarization shift (PDS) - abnormal prolonged depolarization observed during interictal activity.112 Cultured astrocytes isolated from the seizure focus of human epileptic tissue possess depolarized resting membrane potentials and are able to generate action potentiallike responses upon injection of current.113 Astrocytes are initially activated by excessive neuronal activity, a potent trigger of astrocyte Ca2+ signaling. However, once activated, neuronal firing is no longer needed for continued astroglial activation. The findings described above suggest that activated astrocytes in the sclerotic hippocampus are not mere bystanders, but active players in epileptogenesis.
Within 24 to 48 hours after seizure induction, activation of GFAP-positive astrocytes is noted throughout the dentate gyrus and hippocampal subfields.107,115 This reactive astrogliosis persists chronically over three to four months.105,114,115 Newly generated astrocytes display distinct changes in glial membrane channels and receptors to promote neuronal hyperexcitability and seizure generation. These changes include GluR1 receptors with elevated flip-to-flop ratios,116 impaired uptake of potassium ion due to inwardly rectifying K+ channels,117 over-expression of adenosine kinase,118 and down-regulation of glutamine synthase, glutamate dehydrogenase, and glial GABA transporter.102
Gene expression of classical inflammatory mediators - the complement pathway, cytokines, and glial markers - is significantly higher in the hippocampus of both juvenile and adult rats after KA-induced seizures, based on microarray analysis in our laboratory (Fig. 2).119 Inflammation-related genes were the single most abundant functional group significantly up-regulated acutely after seizure, and significant elevations persisted even 10 days after SE. In juvenile rats, the acute inflammatory response appears to be part of the homeostatic response, because there is no cell death and no recurrent seizures. Experiencing seizures and an acute inflammatory reaction are not without consequence, however, and the rats become permanently more seizure-susceptible. On the other hand, the inflammatory response in adult rats appears to be chronic, excessive, and persistent, and is accompanied by seizure-induced cell death and subsequent development of spontaneous recurrent seizures only in older animals. Such age-dependent increases are reflected as microglial activation in the hippocampi.98,99
Experimentally induced seizures in rodents trigger a prompt inflammatory response in brain areas recruited in the onset and propagation of epileptic activity.90 Direct intra-cerebral injection of a cytokine worsens seizure activity,120,121 and cytokine receptor antibodies, such as IL-1-receptor antagonist, show powerful anticonvulsant activity.122
The mechanisms by which cytokines lead to neuronal excitability have been explored in several studies focusing on ictogenic and neurotoxic properties of IL-1β mediated by IL-1 receptor (IL-1RI).120,123 IL-1RI colocalizes with NMDA receptors on hippocampal pyramidal neurons, and IL-1RI-mediated modulation of glutamatergic transmission may contribute to excitotoxicity and spontaneous seizures.124 IL-1β binding to its receptor increases NMDA receptor-mediated Ca2+ influx and surface expression of AMPA receptors.125,126 IL-1β acts on astrocytes to inhibit reuptake of glutamate127 and increase glutamate release via TNF-α production,128 both resulting in elevated extracellular glutamate levels and hyperexcitability. Furthermore, IL-1β can stimulate IL-6 release.108 Transgenic mice over-expressing astrocyte IL-6 show markedly increased astrogliosis and microgliosis, a loss of inhibitory interneurons, and are exquisitely and selectively sensitive to glutamatergic agonists.129
Cytokines are involved in the death of neurons. Direct intraventricular IL-1β injection concurrent with brain insult increases injury-induced cell death and brain edema.130,131 Inhibition of IL-1 signaling by IL-1 receptor antagonist injection just prior to chemoconvulsant administration significantly attenuates subsequent hippocampal cell loss, implicating endogenous IL-1 in seizure-associated cell death.132
Cytokines can influence the activation of astrocytes and microglia. Astrogliosis can be induced in healthy animals by the injection of cytokines such as IL-1,133 and injury-induced microglial activation is suppressed in TNF receptor-knockout mice.134 In turn, reactive microglia and astrocytes themselves provide a rich source of cytokines after injury or insult, especially IL-6, TGF-β, LIF and IL-1.135
Transgenic or knock-out mice technology provides a powerful tool for studying genetic control of synaptic excitability and epilepsy. It is relatively easy to insert, delete, or mutate a gene of interest or to study the function of spontaneous mutation of genes causing epilepsy. A chronic brain inflammatory state has been induced in transgenic mice overexpressing specific cytokines to study the functional outcome of inflammatory mediators in the brain.
Transgenic mice with chronic IL-6 production from astrocytes using the transcriptional control of the GFAP promoter display astrogliosis, microgliosis, impaired development of the BBB, and increased levels of acute-phase proteins and proinflammatory cytokines.136,137 These mice develop progressive neurodegeneration in the hippocampus and cerebellum, which in turn contributes to a clinical picture of ataxia, tremor, progressive learning deficits, hippocampal excitability, and spontaneous seizures.137-139 GFAP-IL-6 mice show markedly enhanced sensitivity to glutamatergic-induced seizures and lethality, and this may relate to a compromise of inhibitory paralbumin- or GABA interneurons.129
In IL-6 null mice, reactive astrogliosis and microgliosis are significantly lower after KA-induced seizure, while morphological hippocampal damage, oxidative stress, and apoptotic neuronal death are greater. IL-6 deficiency impairs the inflammatory response after KA-induced seizures and increases neuronal injury.140 IL-6 is a major inducer of metallothionein I and II (MT-I+II), antioxidants and neuroregenerative factors in the CNS.. Decreased MT-I+II levels in IL-6 null mice may contribute to greater oxidative stress and cell death. These findings support the beneficial and protective role of the physiological inflammatory reaction and caution against potential exacerbation of neuronal injury by complete obliteration or blockage of the inflammatory cascade.
Transgenic mice deficient in IL-1β receptor 1 are resistant to experimental febrile seizure. Moreover, intracerebral injection of high dose IL-1β, sufficient to generate limbic seizures in wild type mice, fails to cause seizures in these mice.38 This finding suggests that IL-1β signaling contributes critically to fever-induced hyperexcitability underlying febrile seizures and may provide a link between hyperthermia and genetic susceptibility to seizure.
Epileptic EL mice, a natural model of human multifactorial idiopathic epilepsy and complex partial seizures, experience about 25 - 30 complex partial seizures with secondary generalization during routine weekly cage changing. Prior to the onset of recurrent seizure activity, the number of GFAP-positive astrocytes is similar in EL and non-epileptic mice at young ages.141 Recurrent seizure activity in EL mice produces a unique type of gliosis where microglial activation is diffuse and widespread throughout the cortical surface, including the hippocampus, while astrocyte activation is specifically localized to the hippocampus accompanied by down-regulation of glial glutamate transporters, but without obvious hippocampal neuronal loss or mossy fiber sprouting.142,143 These findings suggest a potential contributory role of glial abnormalities in the recurrent seizure activity of human multifactorial idiopathic epilepsy.
Increased expression of proinflammatory molecules has been demonstrated in brain tissue from patients who received surgery for drug resistant epilepsies.99,144-147 Of note, inflammatory reactions occur not only in classic inflammatory epilepsy such as Rasmussen's encephalitis, but also in common intractable epilepsy that does not typically invoke an inflammatory pathophysiology, such as temporal lobe epilepsy or tuberous sclerosis. Brain inflammation may be a common factor contributing or predisposing to the occurrence of seizures and cell death in various forms of epilepsy of different etiologies.148
Acute inflammatory reaction after seizures has long been suspected due to clinical observations. Mean peripheral blood and CSF-leukocyte counts as well as C-reactive protein, an acute phase reactant, are significantly elevated in patients who present with new onset generalized convulsions without any clinical evidence of CNS or systemic infection.10 Involvement of the "cytokine network" has also been implicated in febrile seizures. Significantly higher plasma IL-6 levels as well as a higher ratio of endogenous IL-1 receptor antagonist to IL-1β are found in children with febrile seizures compared to febrile children without seizures36 and in epilepsy patients with recent tonic-clonic convulsions.149-151
Cytokine gene polymorphism has been linked to epilepsy susceptibility. The increased frequency of a biallelic polymorphism in the promoter region of IL-1β at the -511 position, which is suggested to have effects of higher IL-1β production, has been reported in patients with temporal lobe epilepsy with hippocampal sclerosis and prolonged febrile convulsions.37 Sporadic simple febrile seizure patients exhibit significantly higher frequencies of IL1β -31C/-511T alleles and homozygotes than controls.152 These results suggest that genetic susceptibility to inflammation may contribute to epileptogenesis.
Active inflammation has been detected not only in prototypical inflammatory epilepsy such as Rasmussen's encephalitis or limbic encephalitis, but also in pharmacoresistant epilepsy of diverse etiologies. Microglial activation and proliferation havebeen demonstrated in surgically resected epileptogenic lesions from adult patients with chronic intractable epilepsy. In human mesial temporal lobe epilepsy, activated microglia are increased over ten fold in the sclerotic hippocampi.153 An abundant population of activated microglial cells are found in glioneuronal lesions associated with chronic epileptic activity,154 in focal cortical dysplasia155 and in cortical tubers of the tuberous sclerosis complex.146 The duration of epilepsy and frequency of seizures prior to surgical resection significantly correlate with the density of activated microglia in focal cortical dysplasia,155 highly epileptogenic gangliogliomas, and dysembryoplastic neuroepithelial tumors (DNET).154 Activation of microglia appears to be an important feature of chronic intractable epilepsy, and microglial proliferation may be functionally related to epileptogenic brain lesions of diverse etiology. In addition, pro-inflammatory cytokines, such as IL-1b and its signaling receptor IL-1R1, and NF-kB are highly expressed by neurons and glia in temporal lobe epilepsy,145 focal cortical dysplasia,155 glioneuronal tumors,154 and tuberous sclerosis complex.146 These results strongly support the involvement of inflammatory and immune reactions in the pathogenesis of human CNS disorders associated with epilepsy.
In our laboratory, we quantified cell death, astrocyte proliferation, microglial activation and cytokine release in brain cortical tissue from 13 children who underwent epilepsy surgery. Patients had intractable epilepsies due to focal cortical dysplasia (6), encephalomalacia (5), Rasmussen's encephalitis (1), or mesial temporal lobe epilepsy (1). Five autopsy patients with no history of seizures or neurological diseases were used as controls. We found marked glial activation and neuroinflammation in epileptogenic cortices from children with intractable epilepsy (Fig. 3). The majority of our patients had mental retardation. Numerous fibrillary astrocytes covered the entire cortex and converged on to blood vessels, neurons, and microglia. Large numbers of neurons and astrocytes displayed DNA fragmentation and the magnitude significantly correlated with seizure frequency. Panlaminar astrocytosis, diffuse microglial activation, and release of proinflammatory cytokines, especially IL-1β, IL-8, IL-12p70 and MIP-1β, were present in the epileptogenic cortices, consistent with chronic neuroinflammatory responses. IL-6 and MCP-1 were significantly higher in patients with a family history of epilepsy, suggesting links between genetic susceptibility to inflammation and epilepsy. Our results also suggest that active neuroinflammation occurs in pediatric epilepsy and may play a common pathogenic role in childhood epilepsies of diverse etiologies.
Accumulating evidence suggests that inflammatory and immune reactions may play an important role in neuronal excitability and epileptogenesis. Chronic brain inflammation may also contribute to intractability of seizures and comorbidity in chronic epilepsy patients. No effective treatments currently exist to protect the brain from seizure-induced cell death and prevent future development of chronic epilepsy. Modulation of inflammatory reactions in the brain and targeting of inflammatory mediators may be effective therapeutic strategies to prevent or limit epileptogenesis in the vulnerable nervous system. Anti-inflammatory therapy may be particularly helpful when given during the latency period shortly after the initial neurologic insult, but prior to the onset of epilepsy, before permanent changes can occur in neuronal aggregates that promote hyperexcitability and seizure spread. The causative role of inflammation in the pathogenesis of epilepsy and neurological sequelae of chronic intractable epilepsy needs to be proven and requires further investigation from both clinical and basic science angles.
Figures and Tables
Fig. 1
Acute activation of microglia after KA-induced seizures in CX3cr1EGFP/+ transgenic mice. (A) Low magnification view of a brain section from a Cx3cr1GFP/+ transgenic mouse showing that all parenchymal microglia are fluorescently labeled green because the Cx3cr1-encoding fractalkine chemokine receptor has been replaced by a green fluorescent protein (GFP) reporter gene. The box points to massively accumulated microglia one week after KA-induced seizures, likely at the area of injury, shown in high magnification view (B). High magnification view of PBS control (C) and KA-induced seizures (D). Within two days after status epilepticus, a nearly two-fold increase in the area of fluorescent cells and significant microglial proliferation were noted in KA-treated mice compared to control littermates. Note the ramified processes and apparent increase in the number of GFP+ microglia.
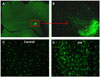
Fig. 2
Time course of changes in expression of select inflammation-related genes significantly regulated in rats after KA at P15 and P30 compared to controls. Genes (C) for transcription factors known to be early mediators of immune and inflammation responses, as well as cytokines associated with microglial activation, are rapidly and dramatically up-regulated after KA-induced seizures at P15 (postnatal day 15; juvenile rats) (A) and P30 (postnatal day 30; adult rats) (B). Note that some of these inflammation-related genes are increased to a greater extent than at P15 and are often persistently elevated beyond 72 hours only in adult rats (P30, B) that show seizure-induced cell death and develop spontaneous recurrent seizures.

Fig. 3
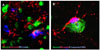
Triple immunofluorescence confocal images of neurons, microglia, and astrocytes in the epileptogenic cortex of patients with chronic intractable childhood epilepsy. (A) Activated microglia (blue) and astrocytes (red) are found in close contact with each other and with neurons (green). Bar = 100µm. (B) Microglial processes (red) wrap around a neuron (green), engulfing fragmented DNA (blue). Bar = 10µm.
References
1. Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994. 35:Suppl 2. S1–S6.

2. Shinnar S, Pellock JM. Update on the epidemiology and prognosis of pediatric epilepsy. J Child Neurol. 2002. 17:Suppl 1. S4–S17.

3. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999. 40:445–452.

4. Hauser WA HD. Epilepsy: Frequency, Causes and Consequences. 1990. New York: Demos.
5. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004. 16:1–13.
6. Neuhaus J, Risau W, Wolburg H. Induction of blood-brain barrier characteristics in bovine brain endothelial cells by rat astroglial cells in transfilter coculture. Ann N Y Acad Sci. 1991. 633:578–580.

7. Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res. 2004. 26:846–853.

8. Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-alpha. Cerebrovasc Brain Metab Rev. 1994. 6:341–360.
9. Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S, et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997. 110:492–499.

10. Rider LG, Thapa PB, Del Beccaro MA, Gale JL, Foy HM, Farwell JR, et al. Cerebrospinal fluid analysis in children with seizures. Pediatr Emerg Care. 1995. 11:226–229.

11. Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000. 20:967–978.

12. deVeber G, Roach ES, Riela AR, Wiznitzer M. Stroke in children: recognition, treatment, and future directions. Semin Pediatr Neurol. 2000. 7:309–317.

14. Kirkham FJ, DeBaun MR. Stroke in Children with Sickle Cell Disease. Curr Treat Options Neurol. 2004. 6:357–375.

15. Aso K, Scher MS, Barmada MA. Cerebral infarcts and seizures in the neonate. J Child Neurol. 1990. 5:224–228.

17. Sreenan C, Bhargava R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term outcome. J Pediatr. 2000. 137:351–355.

18. Golomb MR, Garg BP, Carvalho KS, Johnson CS, Williams LS. Perinatal stroke and the risk of developing childhood epilepsy. J Pediatr. 2007. 151:409–413. 413.e1–413.e2.

19. Yang JS, Park YD, Hartlage PL. Seizures associated with stroke in childhood. Pediatr Neurol. 1995. 12:136–138.

20. Lanska MJ, Lanska DJ, Horwitz SJ, Aram DM. Presentation, clinical course, and outcome of childhood stroke. Pediatr Neurol. 1991. 7:333–341.

21. Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S, et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995. 26:1393–1398.

22. Cutler SM, Pettus EH, Hoffman SW, Stein DG. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp Neurol. 2005. 195:423–429.

23. Lee T, von Scheven E, Sandborg C. Systemic lupus erythematosus and antiphospholipid syndrome in children and adolescents. Curr Opin Rheumatol. 2001. 13:415–421.

24. Toubi E, Khamashta MA, Panarra A, Hughes GR. Association of antiphospholipid antibodies with central nervous system disease in systemic lupus erythematosus. Am J Med. 1995. 99:397–401.

25. Liou HH, Wang CR, Chen CJ, Chen RC, Chuang CY, Chiang IP, et al. Elevated levels of anticardiolipin antibodies and epilepsy in lupus patients. Lupus. 1996. 5:307–312.

26. Watemberg N, Greenstein D, Levine A. Encephalopathy associated with Hashimoto thyroiditis: pediatric perspective. J Child Neurol. 2006. 21:1–5.

28. Joseph FG, Scolding NJ. Neuro-Behçet's disease in Caucasians: a study of 22 patients. Eur J Neurol. 2007. 14:174–180.

29. Mantegazza R, Bernasconi P, Baggi F, Spreafico R, Ragona F, Antozzi C, et al. Antibodies against GluR3 peptides are not specific for Rasmussen's encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J Neuroimmunol. 2002. 131:179–185.

30. Peltola JT, Haapala A, Isojärvi JI, Auvinen A, Palmio J, Latvala K, et al. Antiphospholipid and antinuclear antibodies in patients with epilepsy or new-onset seizure disorders. Am J Med. 2000. 109:712–717.

31. Eriksson K, Peltola J, Keränen T, Haapala AM, Koivikko M. High prevalence of antiphospholipid antibodies in children with epilepsy: a controlled study of 50 cases. Epilepsy Res. 2001. 46:129–137.

32. Yoshimura K, Konishi T, Kotani H, Wakiguchi H, Kurashige T. Prevalence of positive anticardiolipin antibody in benign infantile convulsion. Brain Dev. 2001. 23:317–320.

33. Shrivastava A, Dwivedi S, Aggarwal A, Misra R. Anti-cardiolipin and anti-beta2 glycoprotein I antibodies in Indian patients with systemic lupus erythematosus: association with the presence of seizures. Lupus. 2001. 10:45–50.

34. Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976. 295:1029–1033.

35. Millichap JG. Studies in febrile seizures. I. Height of body temperature as a measure of the febrile-seizure threshold. Pediatrics. 1959. 23(1 Pt 1):76–85.
36. Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002. 26:192–195.
37. Kanemoto K, Kawasaki J, Yuasa S, Kumaki T, Tomohiro O, Kaji R, et al. Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia. 2003. 44:796–799.

38. Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005. 57:152–155.

39. Hall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994. 331:432–438.

40. Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001. 108:E63.

41. Asano Y, Yoshikawa T, Suga S, Kobayashi I, Nakashima T, Yazaki T, et al. Clinical features of infants with primary human herpesvirus 6 infection (exanthem subitum, roseola infantum). Pediatrics. 1994. 93:104–108.

42. Jee SH, Long CE, Schnabel KC, Sehgal N, Epstein LG, Hall CB. Risk of recurrent seizures after a primary human herpesvirus 6-induced febrile seizure. Pediatr Infect Dis J. 1998. 17:43–48.

43. Bertolani MF, Portolani M, Marotti F, Sabbattini AM, Chiossi C, Bandieri MR, et al. A study of childhood febrile convulsions with particular reference to HHV-6 infection: pathogenic considerations. Childs Nerv Syst. 1996. 12:534–539.

44. Kondo K, Nagafuji H, Hata A, Tomomori C, Yamanishi K. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis. 1993. 167:1197–1200.

45. Kawada J, Kimura H, Ito Y, Hara S, Iriyama M, Yoshikawa T, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis. 2003. 188:690–698.

46. Masuyama T, Matsuo M, Ichimaru T, Ishii K, Tsuchiya K, Hamasaki Y. Possible contribution of interferon-alpha to febrile seizures in influenza. Pediatr Neurol. 2002. 27:289–292.
47. Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004. 292:351–357.
48. Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001. 345:656–661.

49. Hirtz DG, Nelson KB, Ellenberg JH. Seizures following childhood immunizations. J Pediatr. 1983. 102:14–18.

50. Contino MF, Lebby T, Arcinue EL. Rotaviral gastrointestinal infection causing afebrile seizures in infancy and childhood. Am J Emerg Med. 1994. 12:94–95.

51. Nishimura S, Ushijima H, Nishimura S, Shiraishi H, Kanazawa C, Abe T, et al. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 1993. 15:457–459.

52. Ushijima H, Bosu K, Abe T, Shinozaki T. Suspected rotavirus encephalitis. Arch Dis Child. 1986. 61:692–694.

53. Saulsbury FT, Winkelstein JA, Yolken RH. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980. 97:61–65.

54. Chen HJ, Chen BS, Wang SF, Lai MH. Rotavirus gastroenteritis in children: a clinical study of 125 patients in Hsin-Tien area. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1991. 32:73–78.
55. Prasad AN, Stafstrom CF, Holmes GL. Alternative epilepsy therapies: the ketogenic diet, immunoglobulins, and steroids. Epilepsia. 1996. 37:Suppl 1. S81–S95.

56. Klein R, Livingston S. The effect of adrenocorticotropic hormone in epilepsy. J Pediatr. 1950. 37:733–742.

57. Hrachovy RA, Frost JD Jr, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. The Journal of pediatrics. 1994. 124(5 Pt 1):803–806.

59. Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000. 294:909–915.
60. Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998. 21:471–476.

61. Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958. 8:435–445.

62. Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007. 62:67–80.

63. Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science. 1994. 265:648–651.

64. Bahi-Buisson N, Villanueva V, Bulteau C, Delalande O, Dulac O, Chiron C, et al. Long term response to steroid therapy in Rasmussen encephalitis. Seizure. 2007. 16:485–492.

65. Cowan LD, Hudson LS. The epidemiology and natural history of infantile spasms. J Child Neurol. 1991. 6:355–364.

66. Cowan LD, Bodensteiner JB, Leviton A, Doherty L. Prevalence of the epilepsies in children and adolescents. Epilepsia. 1989. 30:94–106.

67. Aicardi J. Aicardi J, editor. Infantile spasms and related syndromes. Epilepsy in children. 1986. New York: Raven Press.
69. Baram TZ. Pathophysiology of massive infantile spasms: perspective on the putative role of the brain adrenal axis. Ann Neurol. 1993. 33:231–236.

70. Low NL. Infantile spasms with mental retardation. II. Treatment with cortisone and adrenocorticotropin. Pediatrics. 1958. 22:1165–1169.
71. Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004. 62:1668–1681.

72. Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996. 97:375–379.

73. Hrachovy RA, Frost JD Jr, Kellaway P, Zion TE. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983. 103:641–645.

74. Lombroso CT. A prospective study of infantile spasms: clinical and therapeutic correlations. Epilepsia. 1983. 24:135–158.

75. Eisermann MM, DeLaRaillère A, Dellatolas G, Tozzi E, Nabbout R, Dulac O, et al. Infantile spasms in Down syndrome--effects of delayed anticonvulsive treatment. Epilepsy Res. 2003. 55:21–27.

78. Yamatogi Y, Ohtsuka Y, Ishida T, Ichiba N, Ishida S, Miyake S, et al. Treatment of the Lennox syndrome with ACTH: a clinical and electroencephalographic study. Brain Dev. 1979. 1:267–276.

79. Landau WM, Kleffner FR. Syndrome of acquired aphasia with convulsive disorder in children. Neurology. 1957. 7:523–530.

80. Sinclair DB, Snyder TJ. Corticosteroids for the treatment of Landau-kleffner syndrome and continuous spike-wave discharge during sleep. Pediatr Neurol. 2005. 32:300–306.

81. Tsuru T, Mori M, Mizuguchi M, Momoi MY. Effects of high-dose intravenous corticosteroid therapy in Landau-Kleffner syndrome. Pediatr Neurol. 2000. 22:145–147.

82. Tassinari CA BM, Dravet C, Dalla Bernardina B, Roger J. Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P, editors. Epilepsy with continuous spikes and waves during slow sleep-otherwise described as epilepsy with electrical status epilepticus during slow sleep (ESES). Epileptic syndromes in infancy, childhood and adolescence. 2002. 3rd ed. London: John Libbey.
83. Gallagher S, Weiss S, Oram Cardy J, Humphries T, Harman KE, Menascu S. Efficacy of very high dose steroid treatment in a case of Landau-Kleffner syndrome. Dev Med Child Neurol. 2006. 48:766–769.

84. Snead OC 3rd, Benton JW, Myers GJ. ACTH and prednisone in childhood seizure disorders. Neurology. 1983. 33:966–970.

85. Oguni H, Hayashi K, Awaya Y, Fukuyama Y, Osawa M. Severe myoclonic epilepsy in infants--a review based on the Tokyo Women's Medical University series of 84 cases. Brain Dev. 2001. 23:736–748.

86. Verhelst H, Boon P, Buyse G, Ceulemans B, D'Hooghe M, Meirleir LD, et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure. 2005. 14:412–421.

87. Plata-Salamán CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, et al. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000. 75:248–258.

88. Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comp Neurol. 1997. 378:482–492.

89. Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004. 16:321–334.

90. Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001. 63:125–149.

92. Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997. 17:3727–3738.

93. Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999. 53:915–921.

95. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996. 19:312–318.

96. Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005. 76:77–98.

97. Taniwaki Y, Kato M, Araki T, Kobayashi T. Microglial activation by epileptic activities through the propagation pathway of kainic acid-induced hippocampal seizures in the rat. Neurosci Lett. 1996. 217:29–32.

98. Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 2003. 14:494–503.

99. Ravizza T, Rizzi M, Perego C, Richichi C, Velísková J, Moshé SL, et al. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia. 2005. 46:Suppl 5. 113–117.

100. Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000. 41:Suppl 2. S23–S30.

101. Henshall DC. Apoptosis signalling pathways in seizure-induced neuronal death and epilepsy. Biochem Soc Trans. 2007. 35(Pt 2):421–423.

102. Kang TC, Kim DS, Kwak SE, Kim JE, Won MH, Kim DW, et al. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia. 2006. 54:258–271.

104. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003. 100:13632–13637.

105. Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003. 182:21–34.

106. Brandt C, Potschka H, Löscher W, Ebert U. N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience. 2003. 118:727–740.

108. Aronica E, Gorter JA, Rozemuller AJ, Yankaya B, Troost D. Interleukin-1 beta down-regulates the expression of metabotropic glutamate receptor 5 in cultured human astrocytes. J Neuroimmunol. 2005. 160:188–194.

109. Tilleux S, Berger J, Hermans E. Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol. 2007. 189:23–30.

110. Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006. 7:194–206.

111. Gloor P. Epilepsy surgery. 1999. New York: Raven Press.
112. Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, et al. An astrocytic basis of epilepsy. Nat Med. 2005. 11:973–981.

113. O'Connor ER, Sontheimer H, Spencer DD, de Lanerolle NC. Astrocytes from human hippocampal epileptogenic foci exhibit action potential-like responses. Epilepsia. 1998. 39:347–354.
114. Jørgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp Neurol. 1993. 120:70–88.

115. Somera-Molina KC, Robin B, Somera CA, Anderson C, Stine C, Koh S, et al. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia. 2007. 48:1785–1800.

116. Seifert G, Schröder W, Hinterkeuser S, Schumacher T, Schramm J, Steinhäuser C. Changes in flip/flop splicing of astroglial AMPA receptors in human temporal lobe epilepsy. Epilepsia. 2002. 43:Suppl 5. 162–167.

117. Schröder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, et al. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia. 2000. 41:Suppl 6. S181–S184.

118. Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rülicke T, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005. 128(Pt 10):2383–2395.

119. Chung HKS. Encyclopedia of Basic Epilepsy Research. Elsevier: Burlington;in press.
120. Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999. 19:5054–5065.

121. Oprica M, Eriksson C, Schultzberg M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J Cell Mol Med. 2003. 7:127–140.

122. Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000. 97:11534–11539.

123. Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, et al. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005. 25:6734–6744.

124. Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008. 29:142–160.

125. Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002. 295:2282–2285.
126. Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003. 23:8692–8700.

127. Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996. 7:2181–2185.
128. Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001. 11:387–394.

129. Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003. 73:176–187.

130. Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Ann N Y Acad Sci. 2003. 992:39–47.
131. Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000. 23:618–625.

132. Panegyres PK, Hughes J. The neuroprotective effects of the recombinant interleukin-1 receptor antagonist rhIL-1ra after excitotoxic stimulation with kainic acid and its relationship to the amyloid precursor protein gene. J Neurol Sci. 1998. 154:123–132.

133. Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci. 1994. 16:128–136.

134. Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996. 2:788–794.

135. Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997. 20:570–577.

136. Brett FM, Mizisin AP, Powell HC, Campbell IL. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J Neuropathol Exp Neurol. 1995. 54:766–775.

137. Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993. 90:10061–10065.

138. Campbell IL. Transgenic mice and cytokine actions in the brain: bridging the gap between structural and functional neuropathology. Brain Res Brain Res Rev. 1998. 26:327–336.

139. Steffensen SC, Campbell IL, Henriksen SJ. Site-specific hippocampal pathophysiology due to cerebral overexpression of interleukin-6 in transgenic mice. Brain Res. 1994. 652:149–153.

140. Penkowa M, Molinero A, Carrasco J, Hidalgo J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience. 2001. 102:805–818.

141. Brigande JV, Wieraszko A, Albert MD, Balkema GW, Seyfried TN. Biochemical correlates of epilepsy in the E1 mouse: analysis of glial fibrillary acidic protein and gangliosides. J Neurochem. 1992. 58:752–760.

142. Drage MG, Holmes GL, Seyfried TN. Hippocampal neurons and glia in epileptic EL mice. J Neurocytol. 2002. 31:681–692.
143. Ingram EM, Wiseman JW, Tessler S, Emson PC. Reduction of glial glutamate transporters in the parietal cortex and hippocampus of the EL mouse. J Neurochem. 2001. 79:564–575.

144. Sheng JG, Boop FA, Mrak RE, Griffin WS. Increased neuronal beta-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1 alpha immunoreactivity. J Neurochem. 1994. 63:1872–1879.

145. Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002. 952:159–169.

146. Maldonado M, Baybis M, Newman D, Kolson DL, Chen W, McKhann G 2nd, et al. Expression of ICAM-1, TNF-alpha, NF kappa B, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol Dis. 2003. 14:279–290.

147. Baranzini SE, Laxer K, Bollen A, Oksenberg JR. Gene expression analysis reveals altered brain transcription of glutamate receptors and inflammatory genes in a patient with chronic focal (Rasmussen's) encephalitis. J Neuroimmunol. 2002. 128:9–15.

148. Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005. 46:1724–1743.

149. Hulkkonen J, Koskikallio E, Rainesalo S, Keränen T, Hurme M, Peltola J. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Res. 2004. 59:199–205.

150. Peltola J, Hurme M, Miettinen A, Keränen T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Res. 1998. 31:129–133.

151. Peltola J, Palmio J, Korhonen L, Suhonen J, Miettinen A, Hurme M, et al. Interleukin-6 and interleukin-1 receptor antagonist in cerebrospinal fluid from patients with recent tonic-clonic seizures. Epilepsy Res. 2000. 41:205–211.

152. Kira R, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, et al. Genetic susceptibility to simple febrile seizures: interleukin-1beta promoter polymorphisms are associated with sporadic cases. Neurosci Lett. 2005. 384:239–244.

153. Beach TG, Woodhurst WB, MacDonald DB, Jones MW. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neurosci Lett. 1995. 191:27–30.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download