Abstract
Anaplastic thyroid carcinoma (ATC) is one of the most malignant human neoplasms and has a grave prognosis. This study gives an update on our experience with this unusual neoplasm, with specific focus on the response to various treatment modalities. Forty-seven patients with histologically proven ATCs were enrolled (19 men, 28 women; mean age, 62.8 years). This number represents 1.5% among a total of 3,088 thyroid cancers treated between 1977 and 2002. The mean tumor diameter was 8.8 cm, and 22 patients had distant metastasis. Extrathyroidal extension was seen in 26 (89.7%) of the cases that underwent surgery. Treatment modalities adopted could be classified into 5 groups: Group 1, biopsy only; Group 2, biopsy and chemoradiotherapy; Group 3, debulking only; Goup 4, debulking and chemoradiotherapy; Group 5, complete excision and chemoradiotherapy. Survival was calculated from the time of diagnosis, and comparisons of survival were done by log-rank analysis. The mean survival was 4.3 months (range, 1.0-21 months). The mean survival based on treatment modalities were as follows: Group 1 (n = 10), 2.1 months, Group 2 (n = 8); 3.6 months; Group 3 (n = 7), 3.0 months; Group 4 (n = 14), 3.5 months, Group 5 (n = 8), 9.4 months. There was no significant difference in survival time between the various types of treatment modalities. Even though a small improvement in survival was observed with complete excision and aggressive multimodality therapy, nearly all ATCs remain unresponsive to ongoing treatment modalities and as such, present a therapeutic dilemma. A more effective treatment regimen should be sought in order to improve survival.
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive and rapidly progressing human carcinomas resulting in fatal prognosis.1 ATCs characteristically manifest suddenly as a rapidly growing thyroid mass, and most are known to arise from a pre-existing malignant or benign thyroid disease.2,3 Surgical approaches are generally not appropriate because this disease usually presents as a huge cancerous mass invading adjacent tissues, with distant metastasis at the time of diagnosis. An aggressive treatment may be attempted for the tumors at an early stage, but curative therapy has been reported to be almost impossible.4 Although the multimodality treatment approach is reported to be necessary for this condition, there are only few reports regarding the efficacy of surgery with adjuvant therapies in ATC.5 Moreover, as the incidence of ATC is very low, there are only a few studies pertaining to its accurate diagnosis and clinical course after therapy.
In this study, the authors report on 47 cases of ATCs over a 26-year period. We describe herein the retrospective analysis of various treatment results after surgery, including multimodal treatment, and ascertain the efficacy of these modalities.
From January 1977 to December 2002, 47 patients with ATC, representing 1.5% of 3,088 total patients with thyroid carcinoma, who underwent surgery in the Department of Surgery, Yonsei University College of Medicine were enrolled in this study. These patients' records were retrospectively analyzed with respect to clinical manifestations, size of tumor, extent of disease, distant metastasis, pathologic findings, treatment modality, and cause of death. The outcome of the multimodal treatment method was also evaluated.
Surgical therapy for ATC was comprised of radical resection for patients with completely resectable tumors, palliative resection for tumor debulking purposes, and excisional biopsy only for unresectable cases.
Adjuvant radiotherapy or radiotherapy as a primary mode of treatment was initiated in 34 patients, and consisted of the conventional 2 Gy per day for a total dose of 50-60 Gy. The hyperfractionated radiation therapy followed the protocol of Kim and Leeper,6 and consisted of administration of low-dose adriamycin (10 mg/m2 per week) given intravenously one and half hours before initiation of radiotherapy, and then 1.6 Gy radiotherapy twice a day at 4-5 hour intervals for a total of 57.6 Gy.
Anticancer chemotherapy was administered in 22 patients as part of the multimodal treatment with radiotherapy, with adriamycin forming the basis of the regimen. Adriamycin only was administered to 15 patients, while the remaining 7 patients received a combination of adriamycin and other chemotherapeutic agents such as 5-FU, cisplatinum, cyclophosphamide, bleomycin, mitomycin, taxol, and retinoic acid.
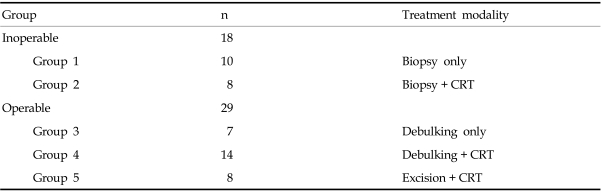
The patients were divided into 5 groups according to the differing modes of therapies they received, and the outcomes were analyzed. The groups were: Group 1, excisional biopsy only; Group 2, chemotherapy and radiation therapy after excisional biopsy only; Group 3, palliative resection only; Group 4, chemotherapy and radiation therapy after palliative resection; and Group 5, chemotherapy and radiation therapy after complete radical resection (Table 1).
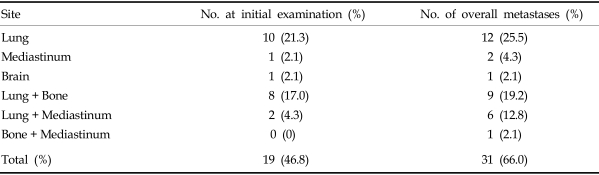
The mean age of the patients at the time of ATC diagnosis was 62.8 years (range, 19-84 years). There were 19 male and 28 female patients, yielding a ratio of 1:1.5. Most patients (46 cases, 97.9%) presented with a rapidly growing anterior neck mass as the initial presenting symptom. In 16 patients (34%) the presenting symptom was sudden enlargement of a previously existing thyroid mass. Two of these patients had a history of surgery for papillary thyroid carcinoma. Other symptoms that were present among the remaining patients were dyspnea, hoarseness, dysphagia, weight loss, and the restriction of neck motion. The mean duration of disease was 2.6 months (range, 15 days-12 months). The mean diameter of the thyroid mass at the time of diagnosis was 8.8 cm (range, 2 - 20 cm). There were 25 patients in whom the lesion was confined to the neck, however, all of them showed invasion into adjacent tissues and nodal metastasis in the neck. Twenty-two patients presented distant metastasis at the time of initial diagnosis. Distant metastases were comprised of 10 cases of lung metastasis, and 8 cases which showed both lung and bone metastases. Two patients demonstrated both lung and mediastinal metastases, and there was 1 case each of brain and mediastinal metastasis.
The most common histological type was giant cell type in 13 patients, followed by 10 cases of spindle cell type, 7 cases of combined giant cell and spindle cell type, and 1 case of epidermoid type histology. In the remaining 16 cases, the precise histological type could not be ascertained. In fourteen patients, ATCs were combined with differentiated thyroid carcinoma, consisting of 6 cases of papillary carcinoma and 6 cases of follicular carcinoma. In one case both papillary and follicular types were present, and the last case showed both papillary and epidermoid components.
Of the 47 total patients, some kind of surgical treatment was possible in 29 (61.7%). Among them, only 8 patients (17.0%) underwent radical surgery, while the remaining 21 (44.7%) underwent palliative surgery, and adjuvant therapies were selected in order to alleviate symptoms such as dyspnea and dysphagea.
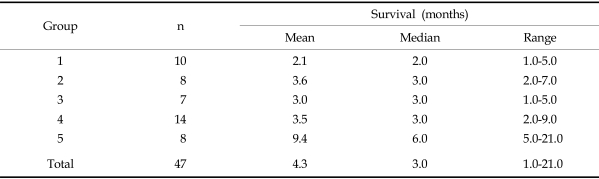
The mean survival time in Group 1, from the time of diagnosis to death, was 2.1 months. For Groups 2, 3, 4, and 5, survival times were 3.6 months, 3.0 months, 3.5 months, and 9.4 months, respectively. A longer survival period was observed in Group 5 than that of Group 1, but there was no statistical difference between the two groups (Table 2).
In Group 5, although the mean survival was 9.4 months, most patients showed relatively short survival times, as the exclusion of 2 cases gave a mean of 4.8 months (range, 1-7 months). The two patients who survived for longer periods didn't show any distinctive clinical features compared to the others.
Overall, most of the patients demonstrated rapid disease progression and distant metastasis, despite the various modes of therapy. The most frequent organ of metastasis was the lung, while frequent metastatic lesions were also observed in the bones and mediastinum (Table 3). The rate of distant metastasis at the time of diagnosis was 46.8%, but this rate increased to 66.0% by the final count.
For all patients, the mean period of survival was 4.3 months (range, 1-21 months), and the most common cause of death was airway obstruction due to locally advanced disease and systemic metastasis in 32 patients, followed by distant metastasis in 14 patients.
Small ATC tumors that are discovered incidentally have been reported to have a more favorable prognosis, and those that are confined to the neck also show a better prognosis compared to tumors with distant metastasis.7,8 However, according to the AJCC staging system, ATCs at any size are assigned to stage IV disease;9 all present frequent metastasis and invasion, despite treatment, leading to death, and thus have a very poor prognosis. Until recently, no definite risk factors for this disease had been identified.10,11
A single mode of therapy, whether it be surgery, chemotherapy or radiotherapy, fails to afford significant, favorable treatment outcomes. While multimodal approaches may enhance treatment responses to a small degree, the implementation of these modalities is often impractical, as many patients are old and unable to tolerate the intensity of the treatments; consequently, their disease progresses rapidly even during therapy.12
As in many other types of carcinomas, radical resection for ATC may be the mainstay as far as mode of therapy, but surgery itself is seldom possible due to extensive local invasion and distant metastasis at the time of diagnosis.8,9,13,14 In this study, radical surgery was performed in only 8 patients (17.0%). There are conflicting data regarding the effect of the extent of surgery for ATC on survival. Some authors have reported improved survival rates in patients with small tumors who were candidates for radical surgery over those for patients who received palliative resection.15-17 Conversely, most other authors are of the opinion that survival is not increased, regardless of the type of surgery undertaken.18,19 Furthermore, even with aggressive surgical therapy for those invasive ATCs, there is no evidence of decreased recurrence rates, while the post-surgical morbidity rates increase.8 Therefore, aggressive surgical resection involving vital organs such as laryngectomy or tracheal resection for locally invasive ATCs are not appropriate. In this report, radical surgical approaches for ATCs with even an early stage of invasion did not produce greater survival benefits compared to other treatment modalities.
One chemotherapeutic agent that seems to demonstrate some anti-cancer effect against ATC is adriamycin, which is more effective when administered in combination than as a single agent, and is also known to act synergistically with radiotherapy.10 A current treatment modality commonly employed is the combination of adriamycin and cisplatin administration with hyperfractionated radiation therapy. There have been a number of reports to date which suggest that the postoperative administration of adriamycin and hyperfractionated radiation therapy increase the local control rate and prolong survival,6 while others have suggested that thyroidectomy after neoadjuvant chemotherapy may be more feasible.10 In contrast, we were not able to observe any benefit of chemotherapy, whether it was done by neoadjuvant or adjuvant means, nor any increase in survival. These results are in agreement with the reports which found no improvement in survival despite the combination of chemotherapy and radiotherapy, and which concluded that the role of chemotherapy, preoperatively or postoperatively is equivocal.20,21
It has been acknowledged that the efficacy of radiotherapy for ATC is largely unsatisfactory, and in particular, conventional radiotherapy has not seemed to have any therapeutic effects at all.19,22 Despite these disappointing results of conventional radiotherapy, hyperfractionated radiation therapy and combined chemotherapy has been suggested as a multimodal treatment approach to improve survival rates,6,15,16 though some disagree.12,22,23 Our study showed a mean survival of only 3.5 months after this combined mutimodal treatment for patients with unresectable disease, while the same adjuvant treatment after radical surgery tended to show improved survival. However, the difference was not significant. These results demonstrate that the combination of hyperfractionated radiation therapy with chemotherapy does not afford significantly improved survival results. The modality may be useful as an adjuvant mode of therapy in improving the outcome for patients after radical resection. However, this has not been confirmed statistically and therefore, further studies and long-term investigation should be done.
Many studies have addressed the question of risk factors for survival in ATC, and have shown that for patients less than 45 years of age with localized disease in the neck, and who receive aggressive surgical therapy such as thyroidectomy followed by radiotherapy and chemotherapy, there is improvement in survival. However, the results of this study showed no significant difference in survival regardless of the types of treatment modalities used, and there was no influence of clinicopathological characteristics. In our series, only 2 patients survived for relatively longer periods of time (11 months, 21 months); these numbers are insufficient to draw any meaningful conclusions.
The dismal treatment results for ATCs have stimulated the investigation of new therapeutic methods with improved outcomes. There have been a number of trials of new materials or therapeutic methods which have already turned out to be ineffective or too toxic to clinically apply, such as exogenous interleukin-6, transfection of human thyroperoxidase gene in tumor cells, and imatineb mesylate monotherapy.24 In recent studies, some trials were partially successful or promising in vitro or vivo. Examples include the use of bone morphogenic protein (BMP-7) by inhibiting cycli-dependent kinase activity, histon deacetyase inhibitors with increased apoptosis, an E1B 55-kDa gene-defective adenovirus (ONYX-015) with adriamycin and paclitaxel, restoration of p53 expression in ATC cells, gene therapy using the interleukin-12 gene in BALB/C (nu/nu) mice, and re-differentiation therapy using retinoids.25-29 Although observations may suggest that some of these methods have a potential therapeutic effect on ATCs or can act as an adjunct to another primary treatment modality, their efficacy and safety have not yet been ascertained in human trials, and further confirmation through in-depth studies is required.
ATCs are accompanied by unfavorable prognosis, irrespective of mode of therapy, and thus present a serious dilemma to the patient as well as the physician. Therefore, detailed, prospective investigations are direly needed to elucidate an effective mode of treatment.
References
1. Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol. 1999; 16:64–69. PMID: 9890741.

2. Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997; 79:564–573. PMID: 9028369.
3. Us-Krasovec M, Golouh R, Auerperg M, Besic N, Ruparcic-Oblak L. Anaplastic thyroid carcinoma in fine needle aspirates. Acta Cytol. 1996; 40:953–958. PMID: 8842172.
4. Demeter JG, De Jong SA, Lawrence AM, Paloyan E. Anaplastic thyroid carcinoma: risk factors and outcome. Surgery. 1991; 110:956–961. discussion 961-3. PMID: 1745983.
5. Tallroth E, Wallin G, Lundwell G, Lowhagen T, Einhorn J. Multimodal treatment in anaplastic giant thyroid carcinoma. Cancer. 1987; 60:1428–1431. PMID: 2441842.
6. Kim JH, Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer. 1987; 60:2372–2375. PMID: 3664425.

7. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg. 2001; 25:617–622. PMID: 11369989.

8. Pacheco-Ojeda LA, Martinez AL, Alvarez M. Anaplastic thyroid carcinoma in ecuador: analysis of prognostic factors. Int Surg. 2001; 86:117–121. PMID: 11918236.
10. Farnebo L, Tash O, Wallin G. vanHeerden JA, editor. Anaplastic giant cell carcinoma of the thyroid. Common problems in endocrine surgery. 1989. London: Yearbook;p. 33–34.
11. Kobayashi T, Asakawa H, Umeshita K, Takeda T, Maruyama H, Matsuzuka F, et al. Treatment of 37 patients with anaplastic carcinoma of the thyroid. Head Neck. 1996; 18:36–41. PMID: 8774920.

12. Haigh PI. Anaplastic thyroid carcinoma. Curr Treat Options Oncol. 2000; 1:353–357. PMID: 12057160.

13. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Geopfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990; 66:321–330. PMID: 1695118.

14. Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995; 17:41–47. discussion 47-8. PMID: 7883548.

15. Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001; 91:2335–2342. PMID: 11413523.

16. Nilsson O, Lindeberg J, Zedenius J, Ekman E, Tennvall J, Blomgren H, et al. Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World J Surg. 1998; 22:725–730. PMID: 9606289.

17. Kim HY, Chung KW, Kim HW, Youn YK, Oh SK. Clinical analysis of anaplastic thyroid carcinoma. J Korean Surg Soc. 2001; 61:142–147.

18. Lu WT, Lin JD, Huang HS, Chao TC. Does surgery improve the survival of patients with advanced anaplastic thyroid carcinoma? Otolaryngol Head Neck Surg. 1998; 118:728–731. PMID: 9591882.

19. Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. 2000; 11:1083–1089. PMID: 11061600.

20. Schlumberger M, Parmentier C, Delisle MJ, Couette JE, Droz JP, Sarrazin D. Combination therapy for anaplastic giant cell thyroid carcinoma. Cancer. 1991; 67:564–566. PMID: 1985750.

21. Tennvall J, Lundell G, Hallquist A, Wahlberg P, Wallin G, Tibblin S. The Swedish Anaplastic Thyroid Cancer Group. Combined doxorubicin, hyperfractionated radiotherapy, and surgery in anaplastic thyroid carcinoma. Report on two protocols. Cancer. 1994; 74:1348–1354. PMID: 8055459.

22. Junor EJ, Paul J, Reed NS. Anaplastic thyroid carcinoma: 91 patients treated by surgery and radiotherapy. Eur J Surg Oncol. 1992; 18:83–88. PMID: 1582515.
23. Lo CY, Lam KY, Wan KY. Anaplastic carcinoma of the thyroid. Am J Surg. 1999; 177:337–339. PMID: 10326855.

24. Rosen IB, Asa SL, Brierley JD. Clark Oh, Duh QY, Kebebew E, editors. Anaplastic carcinoma of the thyroid gland. Textbook of Endocrine Surgery. 2005. 2nd ed. Philadelphia: W.B.Saunders;p. 159–167.

25. Franzen A, Heldin NE. BMP-7 induced cell cycle arrest of anaplastic thyroid carcinoma via p21(CIP1) and p27 (KIP1). Biochem Biophys Res Commun. 2001; 285:773–781. PMID: 11453659.
26. Greenberg VL, Wiliams JM, Cogswell JP, Mendenhall M, Zimmer SG. Histone deacetylase inhibitors promote apoptosis in differential cell cycle arrest in anaplastic thyroid cells. Thyroid. 2001; 11:315–325. PMID: 11349829.
27. Shi Y, Parhar RS, Zou M, Baitei E, Kessie G, Farid NR, et al. Gene therapy of anaplastic thyroid carcinoma with single-chain interleukin-12 fusion protein. Hum Gene Ther. 2003; 14:1741–1751. PMID: 14670125.
28. Simon D, Koehrle J, Reiners C, Boerner AR, Schmutzler C, Mainz K, et al. Redifferentiation therapy with retinoids: therapeutic option for advanced follicular and papillary thyroid carcinoma. World J Surg. 1998; 22:569–574. PMID: 9597930.

29. Gruning T, Tiepolt C, Zophel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer-does it hold its promise? Eur J Endocrinol. 2003; 148:395–402. PMID: 12656659.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download