Abstract
Retroperitoneal cystic lymphangioma is a rare congenital malformation. The majority of lymphangiomas are present at birth and nearly all present before the age of two years. We report a case of giant cystic retroperitoneal lymphangioma in a patient who first presented with symptoms at the age of 7, underwent surgery, and who then suffered a recurrent mass 11 years later.
Abdominal cystic lymphangiomas are rare benign tumors of the lymphatic vessels.1 They are characterized by the appearance of uni- or multi-septate cystic masses.2 The incidence of cystic lymphangioma is approximately 1/6000 live born and localization of the retroperitoneum is less than 5%.3 More than half (50-65%) of neck and axillar lymphangiomas are present at birth and 90% present symptoms before the age of two years.4 Cystic lymphangiomas of the retroperitoneum are frequently found in older children or adults.5 Although the lesion is benign in nature, it causes pressure on vital organs by mass effect and can therefore cause symptoms. Here, we present a case of a giant retroperitoneal cystic lymphangioma which first presented with symptoms in a 7-year-old who underwent surgery and who returned with a recurrent mass 11 years later.
An 18-year-old male was admitted with epigastric and left upper quadrant pain. Distention due to an abdominal mass was detected on physical examination. The patient had also suffered from abdominal distension, abdominal pain, nausea and vomiting 11 years previously and sonographic findings revealed a hypoechoic mass with smooth margins which lay between the liver hilus and splenic hilus. Laparotomic measurement of the mass, which contained multiple cysts, was 30×20 cm. It was localized between the stomach and transverse colon and filled the bursa omentalis. During laparotomy, the cysts and bursa omentalis were drained, the vessels and capsule of cysts were excised and capitonnage were performed. The cysts contained 2.5 liters of yellow, transudative fluid. Microscopically, the tumor consisted of dilate, large, lymphatic channels of different sizes growing in loose connective tissue. These channels were lined by flattened endothelium. A few bundles of smooth muscle were present in the walls (pathologic number: B-1895/92).
In the current hospitalization, ultrasound examination (US) showed an anechoic multicystic mass of 14×15×20 cm with thick septations. There were echogenic areas within the lesion consistent with calcifications (Fig. 1).
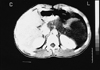
On CT following the administration of contrast material, there was a non-enhancing mass lesion having low attenuation values (+ 12 HU) while including hyperdense calcific foci. The mass surrounded the body and tail of the pancreas and extended to the right side of the midline. It also had extensions into left renal spaces on its posterior aspect, and thereby created a mass effect. The internal septations that were observed on US could not be identified by CT. The body and tail of the pancreas were displaced to the anterior due to compression from the mass. The mass extended to the abdominal wall on the left anterior region (Fig. 2).
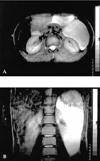
In order to obtain multiplanar views and identify the accompanying lesions, magnetic resonance imaging (MRI) was performed. The lesion was hypointense on T1 weighted and hyperintense on T2 weighted sequences and extended down to the neighborhood of the gall bladder (Fig. 3).
Laparotomic findings revealed a cystic lesion which contained viscous yellow fluid and lay between the tail of the pancreas and the left lobe of the liver.
Together with the previous history and US, CT and MR findings this case was evaluated as recurrent cystic lymphangioma. Postoperative pathological examination confirmed the radiological diagnosis (Fig. 4).
Lymphangiomas are lesions of lymphatic vessels that present as hemangiomas and it is difficult to evaluate whether they are real tumors like hamartomas or only lymphangiectasis.6 However, it is currently accepted that lymphatic vessel tumors are developmental malformations rather than being real tumors.7 Abnormal lymphatic channels dilate in an attempt to produce uni- or multi-locular cystic masses. The opacification of the locules during lymphangiography also supports this hypothesis.8
The most common location is the neck (cystic hygroma) in 75% of the cases, followed by the axilla in 20%. In the remaining 5%, other areas of the body are affected. Abdominal lymphangiomas most commonly occur in the intestinal mesentery, retroperitoneum being the second location of choice.9 Multisystem involvement is very rare and has a bad prognosis. Complications include anemia, hemorrhage, infection, torsion, volvulus and rupture of the mass and intestinal and ureteric obstructions.10
Lymphangiomas have been classified into three groups: capillary (or simple), cavernous and cystic, depending on the size of the lymphatic spaces. Simple lymphangiomas are composed of small, thin-walled, lymphatic channels with considerable connective tissue stroma. Cavernous lymphangiomas consist of dilated lymphatic channels, whereas cystic lymphangiomas contain single or multiple cystic masses.1,5,11
These tumors can recur.12 Recurrence of mass 13 years after the first operation in a 52-year-old female has been reported only once in the literature before.1 The cause of recurrence is unclear but may be due to incomplete resection of the mass. Long term follow-up is needed to determine the exact reason and incidence of recurrence.
The differential diagnosis possibilities are limited for large, cystic, intraabdominal masses of childhood. These are ovarian masses such as teratoma, serous cystadenoma and mucinous cystadenoma. The most commonly encountered ones are the teratomas that demonstrate bone and calcific foci on US and radiographs. Pancreatic pseudocysts, multiloculated cystic nephromas, and liver and spleen cysts originate from the cited organs. Smaller lesions such as traumatic hematomas and urinomas can be clinically diagnosed. In enteric duplications, US can generally demonstrate the significant muscular and mucosal layers and the hyperechogenicity at the periphery of the mass.7 Abdominal lymphangiomas are often discussed in conjunction with mesenteric cysts, but lymphangioma presents earlier in life.13 Nevertheless, the presentation age of our case contradicted with this classical information. Our case was different in the sense that it first presented at the age of 7 years, showed a recurrence after 11 years and had a mass in the retroperitoneum, which is a very rare location. The cyst contents of the present case did not show enhancement after postcontrast images, similar to that reported in a study by Bonhomme et al,14 but in contrast we could not obtained fibrous capsula.
Though numerous publications have stated that lymphangiomas of the retroperitoneum do not calcify, at least two prior case reports have demonstrated calcification.10,15 The series by Davidson et al. includes two examples of unicameral lymphangiomas with a thick layer of mural calcium.8
US, CT and MR appear to be complementary in the evaluation of cystic lymphangiomatosis. US is useful in demonstrating the internal structure of lymphangiomas, particularly septations.16 CT may help distinguish retroperitoneal and mesenteric lymphangiomas from adjacent bowel loops. CT may also be able to distinguish parapelvic renal lymphangiomatosis from hydronephrosis. The ability of MR to provide images in multiple planes without loss of resolution may demonstrate additional lesions and further delineate their boundaries.1
The final diagnosis of lymphangioma is achieved by pathological examination of the specimen after surgical or laparoscopic examination17 and is based on well-established criteria.18 These include a well circumscribed, cystic lesion with or without endothelial lining; a stroma composed of a meshwork of collagen and fibrous tissue; and a wall containing focal aggregates of lymphoid tissue.6,19
Total surgical resection, when possible, is recommended to avoid superinfection, progressive growth, rupture or bleeding.1,17
In conclusion, retroperitoneally located lymphangiomas are rare pathologies of the lymphatic vessels. Although they are benign lesions, postoperative recurrence can occur. US follow-ups at certain intervals can be beneficial in the identification of any recurrence that might develop.
Figures and Tables
Fig. 1
Transverse sonogram of the left flank demonstrating a large mass with multiloculated, thick septum and having hyperechogenic calcific foci (arrow).
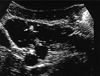
Fig. 2
Transaxial abdominal CT scan demonstrating low attenuation cysts of the retroperitoneum displacing the pancreas (arrow) anteriorly and mildly compressing the adrenal and left kidney. Calcification is seen as high attenuation foci in the cyst.
References
1. Cutillo DP, Swayne LC, Cucco J, Dougan H. CT and MR imaging in cystic abdominal lymphangiomatosis. J Comput Assist Tomogr. 1989. 13:534–536.
2. Devesa R, Munoz A, Torrents M, Carrera JM. Prenatal ultrasonographic findings of intra-abdominal cystic lymphangioma: a case report. J Clin Ultrasound. 1997. 25:330–332.
3. Dahnert W. Radiology review manual. 1993. 2nd ed. Arizona: Williams & Wilkins.
4. Bill AH Jr, Summer DS. A unified concept of lymphangioma and cystic hygroma. Surg Gynecol Obstet. 1965. 120:79–86.
5. Munechika H, Honda M, Kushihashi T, Koizumi K, Gokan T. Computed tomography of retroperitoneal cystic lymphangiomas. J Comput Assist Tomogr. 1987. 11:116–119.
6. Enzinger FM, Weis SW. Tumors of lymph vessels. Soft Tissue Tumors. 1995. St. Louis: Mosby-Years Book;679–700.
7. Mentzel HJ, Schramm D, Vogt S, Reuter A, Mentzel T, Kaiser WA. Intra-abdominal lymphangioma in a newborn. J Clin Ultrasound. 1998. 26:320–322.
8. Davidson AJ, Hartman DS. Lymphangioma of the retroperitoneum: CT and sonographic characteristic. Radiology. 1990. 175:507–510.
9. Fonkalsrud EW. Congenital malformations of the lymphatic system. Semin Pediatr Surg. 1994. 3:62–69.
10. Thomas AM, Leung A, Lynn J. Abdominal cystic lymphangiomatosis: report of a case and review of the literature. Br J Radiol. 1985. 58:467–469.
11. VanCauwelaert P, Gruwez JA. Experience with lymphangioma. Lymphology. 1978. 11:43–48.
12. Heether J, Whalen T, Doolin E. Follow-up of complex unresectable lymphangiomas. Am Surg. 1994. 60:840–841.
13. Kosir MA, Sonnino RE, Gauderer MW. Pediatric abdominal lymphangiomas: a plea for recognition. J Pediatr Surg. 1991. 26:1309–1313.
14. Bonhomme A, Broeders A, Oyen RH, Stas M, De Wever I, Baert AL. Cystic lymphangioma of the retroperitoneum. Clin Radiol. 2001. 56:156–158.
15. Takiff H, Calabria R, Yin L, Stabile BE. Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch Surg. 1985. 120:1266–1269.
16. Blumhagen JD, Wood BJ, Rosenbaum DM. Sonographic evaluation of abdominal lymphangiomas in children. J Ultrasound Med. 1987. 6:487–495.
17. Targarona EM, Moral A, Sabater L, Martinez J, Luque P, Trias M. Laparoscopic resection of a retroperitoneal cystic lymphangioma. Surg Endosc. 1994. 8:1425–1426.
18. Koshy A, Tandon RK, Kapur BM, Rao KV, Joshi K. Retroperitoneal lymphangioma. A case report with review of the literature. Am J Gastroenterol. 1978. 69:485–490.
19. Yunyongying Y, Tang CK, Bruce WG. Solitary cystic lymphangioma of the retroperitoneum. J Urol. 1977. 118:388–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download