Abstract
Our objective was to assess the clinical factors that would reliably distinguish methicillin-resistant S. aureus (MRSA) from methicillin-susceptible S. aureus (MSSA) endocarditis. A retrospective cohort study of clinical features and mortality in patients with MRSA and MSSA endocarditis between March 1986 and March 2004 was performed in a 750-bed, tertiary care teaching hospital. A total of 32 patients (10 MRSA [31.3%] vs 22 MSSA [68.7%]) were evaluated. Their mean age and sex ratio (male/female) were as follows: 30.8 ± 16.0 vs 24.4±19.6 years old and 6/4 vs 13/9, for MRSA and MSSA infective endocarditis (IE), respectively. Univariate and multivariate analyses revealed that persistent bacteremia was significantly more prevalent in MRSA IE (OR, 10.0 [1.480-67.552]; p, 0.018). There was a higher mortality trend for MRSA IE (50.0%) than for MSSA IE (9.1%) (p=0.019). However, persistent bacteremia was not associated with higher mortality (p>0.05). These results indicate that if persistent bacteremia is documented, the likelihood of MRSA endocarditis should be viewed as high, and the patient's antistaphylococcal therapy should be prolonged and/or changed to a more "potent" regimen.
Infective endocarditis (IE) is a serious septic disease that can be life-threatening, unless effective therapy is initiated under the correct diagnosis. Although there have been many developments and improvements in diagnostic and therapeutic methods, the clinical diagnosis of infective endocarditis remains sometimes difficult and the annual incidence, morbidity, and mortality are still relatively high.1,2
Among IE-causing pathogens, S. aureus endocarditis has a higher mortality than endocarditis caused by most other microorganisms.1,3-12 Overall mortality associated with S. aureus bacteremia ranges from 11.9%13 to 46.5%.14 Age, co-morbidity, shock, inappropriate empirical therapy, bacteremia acquired in intensive care unit (ICU) and severe sources of infection may be independent predictors for mortality.15
Since the 1980s, the incidence of S. aureus bacteremia has increased due to the extensive use of indwelling intravenous catheters.16,17 Predisposing factors for methicillin-resistant S. aureus (MRSA) infection include severe underlying conditions, prolonged hospital stay, previous antibiotic treatment, and nasal MRSA carriage.16,18
Since nosocomial S. aureus bacteremias are increasingly due to MRSA, it is important to identify clinical features of endocarditis that can help distinguish between MRSA and MSSA infections. Few data exist comparing the relative rates and clinical characteristics of endocarditis due to MRSA and MSSA.
The aim of this study was to assess clinical factors that would reliably distinguish between MRSA and MSSA endocarditis in order to quickly and properly manage MRSA IE.
We performed a retrospective cohort study identifying clinical features and mortality in patients with MRSA and MSSA endocarditis between March 1986 and March 2004 at the Yongdong Severance hospital in the Republic of Korea. The facility was a 750-bed university-affiliated tertiary care hospital. All patients with at least 2 positive blood cultures for S. aureus and who met the 1994 Duke criteria and modification19 were entered into a database.
Nosocomial endocarditis was defined as endocarditis acquired more than 3 days after the hospital admission date with no clinical manifestations of endocarditis before admission. Persistent bacteremia was defined as the presence of positive blood cultures for S. aureus after ≥ 3 days of empirical anti-staphylococcal antibiotic therapy. Prior antibiotic use and start of antibiotic within 24 h were considered present when an antibiotic was administered for > 48 h within 2 weeks prior to the first positive blood culture and when an antibiotic was started within 24 h of the onset of symptoms.
Microbiological and antimicrobial susceptibility results were obtained from the clinical microbiology database. S. aureus isolates were identified by the growth of coagulase- and catalase-positive gram-positive cocci. Methicillin resistance was determined by the lack of inhibition of growth by an oxacillin disc on mannitol salt agar, according to the criteria of the National Committee for Clinical Laboratory Standards.20
Each patient's hospital chart was retrospectively reviewed. The factors assessed included the patients' demographics, the duration of fever, the origin of infection (unknown, skin, CSF, dental, bone, joint, urinary), whether there was a community or nosocomial source of infection, the presence of heart disease (native or prosthetic valve, prior endocarditis, congenital heart disease, rheumatic heart disease, pacemaker implantation), co-morbidity (diabetes mellitus, liver cirrhosis, neurologic disease, pregnancy, trauma, burn, acute renal failure [ARF]), vegetation (right/left), shock, secondary metastatic infection, atrioventricular [AV] block, prior antibiotic use, start of antibiotic within 24 h, mechanical ventilation, and cardiac operation, laboratory findings (white blood cell count [WBC], creatinine, persistent bacteremia [≥ 3 days]), and mortality.
Univariate analyses using a χ2 test or an independent t test were carried out to determine the risk factors for MRSA IE. Multivariate analysis was performed to assess the independence of the statistically significant variables in univariate analysis, using a multiple logistic regression model. A p value < 0.05 was considered significant. The SPSS version 11.0 statistical software package for Windows was used for all statistical analyses.
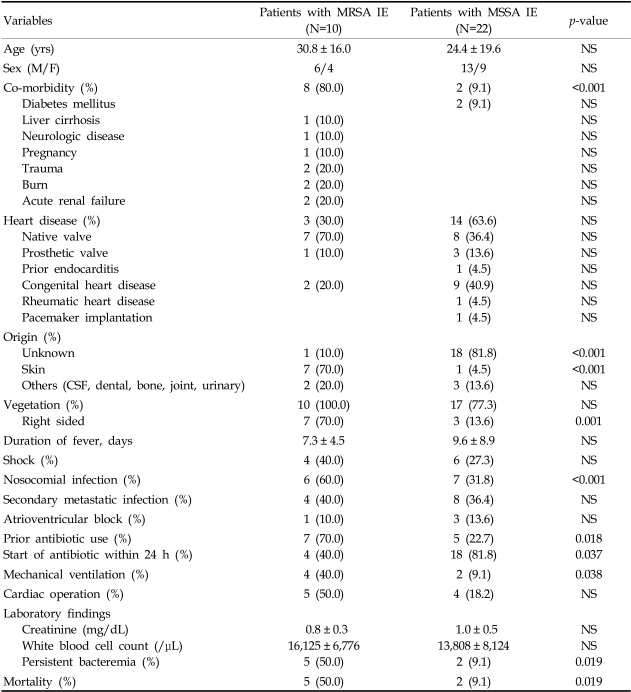
A total of 32 patients met the aforementioned criteria and were included in this study. Of these patients, 10 (31.3%) had MRSA bacteremia and 22 (68.7%) had MSSA bacteremia. Their mean age and sex ratio (male/female) are as follows: 30.8 ± 16.0 vs 24.4 ± 19.6 years old and 13/9 vs 6/4 for MRSA and MSSA infections, respectively. Other demographic and clinical characteristics for these MRSA- and MSSA-infected patients are shown in Table 1.
Compared with the MSSA-infected patients, those with MRSA IE had more infections of skin origin (cellulitis), an increased presence of comorbidity, right sided vegetation, nosocomial infection, persistent bacteremia, mechanical ventilation, prior antibiotic use, and a higher rate of mortality (p < 0.05). MSSA-infected patients were more likely to have begun antibiotics within 24 h of the onset of symptoms and to have an unknown site of origin for the infection (p < 0.05). No statistically significant differences were noted for age, sex, the duration of fever, the presence of heart disease (native or prosthetic valve, prior endocarditis, congenital heart disease, rheumatic heart disease, pacemaker implantation), shock, secondary metastatic infection, AV block, and cardiac operation, and laboratory findings (WBC, creatinine).
Factors demonstrating an association with MRSA endocarditis by univariate analyses were evaluated in a multiple logistic regression model. Statistically significant associations were found only with the presence of persistent bacteremia (odds ratio [OR], 10.0; 95% confidence intervals [CI], 1.480-67.552; p, 0.018). There was a trend of higher mortality for MRSA IE (50.0%) than for MSSA IE (9.1%) (p=0.019). However, persistent bacteremia itself was not associated with higher mortality (p > 0.05).
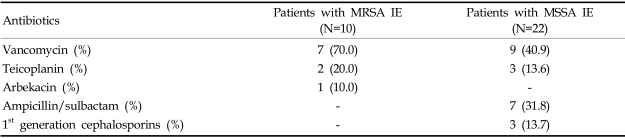
We used a univariate analysis to analyze the effects of an empirical antibiotic regimen (Table 2) on persistent bacteremia. The use of vancomycin was associated with persistent bacteremia, but this association was not significant (p=0.075).
MRSA endocarditis represented only 3% (16/559) of S. aureus infections in 9 large series of retrospective reviews of endocarditis reported before 1998.10,21-26 In a recent review of endocarditis cases at Duke University Medical Center from 1993 to 1999, S. aureus was the etiologic agent in 16% of Staphylococcal endocarditis cases, with most cases occurring in the last 3 years of the study.27 In a survey of infective endocarditis in Japan during 2000 and 2001,28 MRSA was found in 23.1% of cases. In the present study, MRSA was the cause of 31% (10/32) of staphylococcal endocarditis, similar to data recently reported by Chang et al.29 These results reveal a current trend of increasing MRSA IE.
Despite continuous efforts to control MRSA transmission in the hospital, MRSA infection remains a major worldwide concern, and the true impact of MRSA infection on morbidity and mortality remains highly controversial. Although a plethora of clinical studies have demonstrated the virulence of MRSA strains, it has been shown that many MRSA strains neither are highly contagious nor possess virulence determinants.30 MRSA strains did not differ from MSSA strains in intra-leukocyte survival or phagocytic destruction, animal lethality studies, or in the production of extra-cellular hemolysins, enzymes, or toxins.31,32 In addition, Hong Kong strains of MRSA and MSSA were found to be equally pathogenic.33
Although some studies,13,18,29,33-42 including a recent meta-analysis of S. aureus bacteremia,43 have found higher mortality rates among patients with MRSA infection than among those infected with MSSA, many studies failed to identify a relationship between MRSA and mortality.14,15,33,44-53 Our study revealed a trend of higher mortality in MRSA IE (50.0%) than in MSSA IE (9.1%) (p=0.019, Table 1). This finding may be due to the increased presence of co-morbidity (80.0% vs. 9.1%) and a nosocomial origin of infection (60.0% vs. 31.8%) in MRSA IE patients, which is consistent with previous findings.15 However, within this interpretation, it is important to consider several confounding factors, including the end points chosen (crude vs. infection-related mortality), major differences in patient populations, preexisting co-morbidities, the severity of S. aureus infection at the time of diagnosis, sites of infection, and high rates of inappropriate empirical antibiotic regimens prescribed for treatment of MRSA infections.14-16,18,33-40,43-52 Accordingly, more sophisticated clinical studies are required to address these concerns.
Generally, MRSA infections are reportedly more frequent after a longer duration of hospitalization,18,33,41 or concomitant with severe underlying disease,33,41,54,55 intra-vascular catheter infection,41,55 wound infection,41 hospitalization in an ICU,55 and after prior antibiotic treatment.33,41,48,55,56 One of the major confounders in studying MRSA and MSSA pathogenicity might be the higher rates of inappropriate empirical antimicrobial treatments prescribed to MRSA-infected patients, which ranged from 8% to 55% in previous studies.15,37,44,46 One retrospective review showed that 75% (6/8) of patients experiencing recurrent S. aureus bacteremia had received prior vancomycin therapy.57 Although not significant in our studies, there was a trend toward higher rates of prior antibiotic use in MRSA infection (70.0% vs. 22.7%). So, it can be assumed that unnecessary use of antibiotics should be avoided.
In a comparison between MRSA and MSSA endocarditis, Abraham reported that community-acquired MSSA bacteremia was the cause of most of the community-acquired S. aureus endocarditis and the nosocomially acquired MRSA bacteremia was the cause of most of the nosocomially acquired S. aureus endocarditis.55 Our results also showed that MRSA endocarditis was more likely to be hospital-acquired (p=0.001, Table 1). In addition, we found that right-sided vegetations were associated with both MRSA IE (p=0.001) and nosocomial infection (p=0.038).
Chang et al. reported that patients with endocarditis due to MRSA were significantly more likely to experience persistent bacteremia than those with endocarditis due to MSSA.29 In our study, persistent bacteremia also occurred significantly more frequently in endocarditis caused by MRSA than by MSSA. In endocarditis, the organisms within vegetations are presumed to be in the lag phase of growth. Hence, it is possible that the delayed response to vancomycin therapy reflects the slow killing by vancomycin of organisms in this lag phase of growth.58 Our study revealed that the use of vancomycin was associated with persistent bacteremia, although not significantly (p=0.075). Thus, it can be reasoned that persistent bacteremia may be one of predictors of MRSA infective endocarditis, with less significant effect of the slow response of vancomycin. Additionally, Lesens et al. reported that sustained bacteremia was significantly associated with a higher frequency of secondary metastatic infection and with a higher frequency of CRP > 100 mg/L.57 However, our results did not show such associations.
Emergence of MRSA as a common pathogen has added new challenges to the management of IE. Vancomycin is the most appropriate antibiotic choice for the treatment of MRSA IE. For the vancomycin-intolerant patient, antibiotic choices should be made on the basis of antimicrobial susceptibilities and the severity of infection. MRSA is also susceptible to quinupristin-dalfopristin and linezolid. Rifampin combinations might be considered in situations such as prosthetic valve endocarditis. Moreover, in one study, a combination of rifampin and ciprofloxacin cured 100% (10/10) of intravenous drug abusers with right-sided S. aureus endocarditis.59 It is practical to use these drugs in combination to prevent the emergence of quinolone resistance during therapy for an S. aureus infection, provided the MRSA strain is susceptible to both fluoroquinolone and rifampin.60 It seems reasonable to add gentamicin for the first 3 to 5 days of therapy to promote a more rapid clearing of bacteremia and minimization of damage to the heart valve, while avoiding the toxic reactions associated with more prolonged courses of aminoglycosides.61
The findings of our study are particularly important because of the increasing incidence of S. aureus bacteremia and the recent increase in frequency of nosocomial S. aureus bacteremia that has resulted in an increased incidence of IE. If persistent bacteremia is documented, the likelihood of MRSA endocarditis should be viewed as high, and as a result, empiric treatment with antibiotics active against MRSA would be justified. In addition, consideration should be given to "optimizing" the patient's antimicrobial regimen (by using either a more "potent" therapy or a combination antibiotic therapy), prolonging the duration of therapy, and/or exploring the advisability of early surgical intervention. In conclusion, our study affirms the importance of persistent bacteremia as a predictor of MRSA infection.
References
1. Nissen H, Nielsen PF, Frederiksen M, Helleberg C, Nielsen JS. Native valve infective endocarditis in the general population: a 10-year survey of the clinical picture during the 1980s. Eur Heart J. 1992; 13:872–877. PMID: 1644074.

2. Netzer ROM, Zollinger E, Seiler C, Cerny A. Infective endocarditis: Clinical spectrum, presentation and outcome: an analysis of 212 cases 1980-1995. Heart. 2000; 84:25–30. PMID: 10862581.

3. Sanabria TJ, Alpert JS, Goldberg R, Pape LA, Cheeseman SH. Increasing frequency of staphylococcal infective endocarditis: experience at a university hospital. Arch Intern Med. 1990; 150:1305–1309. PMID: 2353863.
4. Manford M, Matharu J, Farrington K. Infective endocarditis in a district general hospital. J R Soc Med. 1992; 85:262–266. PMID: 1433086.
5. Skehan JD, Murray M, Mills PG. Infective endocarditis: incidence and mortality in the north east Thames region. Br Heart J. 1988; 59:62–68. PMID: 3342151.

6. Cho BC, Lee JH, Park JW, Hong CS, Kim JM, Kang SM, et al. Subacute bacterial endocarditis associated with upper endoscopy. Yonsei Med J. 2004; 45:936–940. PMID: 15515208.

7. Chong Y, Lim HS, Lee SY, Cho SY. Lactobacillus casei subspecies casei endocarditis - a case report. Yonsei Med J. 1991; 32:69–73. PMID: 1908610.
8. Chong Y, Yoon KJ, Lee SY, Chung NS. Erysipelothrix rhusiopathiae endocarditis - a case report. Yonsei Med J. 1986; 27:239–243. PMID: 3798967.
9. Chong Y, Kim TS, Lee SY, Shim WH, Choo BK. Cardiobacterium hominis endocarditis - a case report. Yonsei Med J. 1985; 26:78–81. PMID: 4072271.
10. Chong Y, Lee KW, Lee SY, Cho SY. Isolation of Actinobacillus actinomycetemcomitans from the blood of a patient with subacute bacterial endocarditis. Yonsei Med J. 1983; 24:54–58. PMID: 6659555.
11. Chong Y, Song KS, Lee SY. Neisseria subflava infections-bacteriological aspects of two cases. Yonsei Med J. 1975; 16:44–49. PMID: 1224640.
12. Chung Y, Lee SY. Vibrio fetus human infection-isolation from a subacute bacterial endocar-ditis case. Yonsei Med J. 1970; 11:126–130. PMID: 5526468.
13. Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997; 24:584–602. PMID: 9145732.

14. Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987; 9:891–907. PMID: 3317734.
15. Soriano A, Martinez JA, Mensa J, Marco F, Almela M, Moreno-Martinez A, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000; 30:368–373. PMID: 10671343.
16. Steinberg JP, Clark CC, Hackman BO. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996; 23:255–259. PMID: 8842259.
17. Darouiche RO, Musher DM. Editorial response: increasing rates of Staphylococcus aureus bacteremia - a medical device is a merit in disguise and methicillin resistance is merely a vice. Clin Infect Dis. 1996; 23:260–261. PMID: 8842260.
18. Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. Mortality associated with nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1995; 21:1417–1423. PMID: 8749626.
19. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000; 30:633–638. PMID: 10770721.

20. National Committee for Clinical Laboratory Standards (NCCLS). NCCLS document M100-S12:22:1. Performance standards for antimicrobial susceptibility testing: 12th international supplement. 2002. Wayne PA: NCCLS.
21. Benn M, Hagelskjaer LH, Tvede M. Infective endocarditis, 1984 through 1993: a clinical and microbiological survey. J Intern Med. 1997; 242:15–22. PMID: 9260562.

22. Espersen F, Frimodt-Moller N. Staphylococcus aureus endocarditis. A review of 119 cases. Arch Intern Med. 1986; 146:1118–1121. PMID: 3718098.
23. Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users: prognostic features in 102 episodes. Ann Intern Med. 1992; 117:560–566. PMID: 1524330.
24. Manolis AS, Melita H. Echocardiographic and clinical correlates in drug addicts with infective endocarditis. Implications in vegetation size. Arch Intern Med. 1988; 148:2461–2465. PMID: 3190378.
25. Watanakunakorn C. Staphylococcus aureus endocarditis at a community teaching hospital, 1980 to 1991. An analysis of 106 cases. Arch Intern Med. 1994; 154:2330–2335. PMID: 7944855.
26. Watanakunakorn C, Burkert T. Infective endocarditis at a large community teaching hospital, 1980-1990. A review of 210 episodes. Medicine. 1993; 72:90–102. PMID: 8479327.
27. Cabell CH, Jollis JG, Peterson GE, Corey GR, Anderson DJ, Sexton DJ, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002; 162:90–94. PMID: 11784225.

28. Nakatani S, Mitsutake K, Hozumi T, Yoshikawa J, Akiyama M, Yoshida K, et al. Current characteristics of infective endocarditis in Japan: an analysis of 848 cases in 2000 and 2001. Circ J. 2003; 67:901–905. PMID: 14578594.
29. Chang FY, MacDonald BB, Peacock JE Jr, Musher DM, Triplett P, Mylotte JM, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine. 2003; 82:322–332. PMID: 14530781.
30. Chang FY. Staphylococcus aureus bacteremia and endocarditis. J Microbiol Immunol Infect. 2000; 33:63–68. PMID: 10917874.
31. Peacock JE, Moorman D, Wenzel RP, Mandell GL. Methicillin-resistant Staphylococcus aureus: microbiological virulence of an epidemiologic strain. J Infect Dis. 1981; 144:575–582. PMID: 6977003.
32. Cutler RR. Relationship between antibiotic resistance, the production of "virulence factors" and virulence for experimental animals in Staphylococcus aureus. J Med Microbial. 1979; 12:55–62.
33. French GL, Cheng AF, Ling JM, Mo P, Donnan S. Hong Kong strains of methicillin-resistant and methicillin-sensitive Staphylococcus aureus have similar virulence. J Hosp Infect. 1990; 15:117–125. PMID: 1969433.
34. Rello J, Torres A, Ricart M, Valles J, Gonzalez J, Artigas A, et al. Ventilator-associated pneumonia by Staphylococcus aureus: comparison of methicillin-resistant and methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994; 150:1545–1549. PMID: 7952612.
35. Mekontso-Dessap A, Kirsch M, Brun-Buisson C, Loisance D. Poststernotomy mediastinitis due to Staphylococcus aureus: comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001; 32:877–883. PMID: 11247711.
36. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002; 162:2229–2235. PMID: 12390067.
37. Talon D, Woronoff-Lemsi MC, Limat S, Bertrand X, Chatillon M, Gil H, et al. The impact of resistance to methicillin in Staphylococcus aureus bacteremia on mortality. Eur J Intern Med. 2002; 13:31–36. PMID: 11836080.
38. Conterno LO, Wey SB, Castelo A. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 1998; 19:32–37. PMID: 9475347.
39. Ibelings MM, Bruining HA. Methicillin-resistant Staphylococcus aureus: acquisition and risk of death in patients in the intensive care unit. Eur J Surg. 1998; 164:411–418. PMID: 9696441.
40. Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003; 36:592–598. PMID: 12594640.
41. Cheng AF, French GL. Methicillin-resistant Staphylococcus aureus bacteremia in Hong Kong. J Hosp Infect. 1988; 12:91–101. PMID: 2905726.
42. Topeli A, Unal S, Akalin HE. Risk factors influencing clinical outcome in Staphylococcus aureus bacteremia in a Tukish University Hospital. Int J Antimicrob Agents. 2000; 14:57–63. PMID: 10717502.
43. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a metaanalysis. Clin Infect Dis. 2003; 36:53–59. PMID: 12491202.
44. Harbarth S, Rutschmann O, Sudre P, Pittet D. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch Intern Med. 1998; 158:182–189. PMID: 9448557.
45. Tumbarello M, De Gaetano Donati K, Tacconelli E, Citton R, Spanu T, Leone F, et al. Risk factors and predictors of mortality of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in HIV-infected patients. J Antimicrob Chemother. 2002; 50:375–382. PMID: 12205062.
46. Roghmann MC. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med. 2000; 160:1001–1004. PMID: 10761966.
47. Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis. 2000; 31:1170–1174. PMID: 11073748.
48. Lewis E, Saravolatz LD. Comparison of methicillin-resistant and methicillin-sensitive Staphylococcus aureus bacteremia. Am J Infect Control. 1985; 13:109–114. PMID: 3849269.
49. Hershow RC, Khayr WF, Smith NL. A comparison of clinical virulence of nosocomially acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus infections in a university hospital. Infect Control Hosp Epidemiol. 1992; 13:587–593. PMID: 1469267.
50. Mylotte JM, Aeschlimann JR, Rotella DL. Staphylococcus aureus bacteremia: factors predicting hospital mortality. Infect Control Hosp Epidemiol. 1996; 17:165–168. PMID: 8708354.
51. Marty L, Flahault A, Suarez B, Caillon J, Hill C, Andremont A. Resistance to methicillin and virulence of Staphylococcus aureus strains in bacteremic cancer patients. Intensive Care Med. 1993; 19:285–289. PMID: 8408938.
52. Selvey LA, Whitby M, Johnson B. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infect Control Hosp Epidemiol. 2000; 21:645–648. PMID: 11083180.
53. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992; 117:390–398. PMID: 1503330.
54. Mizushima Y, Kawasaki A, Hirata H, Daimon Y, Oosaki R, Morinaga S, et al. An analysis of bacteremia in a university hospital in Japan over a 10-year period. J Hosp Infect. 1994; 28:285–298. PMID: 7963471.
55. Pujol M, Pena C, Pallares R, Ayats J, Ariza J, Gudiol F. Risk factors for nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994; 13:96–102. PMID: 8168571.
56. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988; 16:128–140. PMID: 2841893.
57. Lesens O, Hansmann Y, Brannigan E, Remy V, Hopkins S, Martinot M, et al. Positive surveillance blood culture is a predictive factor for secondary metastatic infection in patients with Staphylococcus aureus bacteraemia. J Infect. 2004; 48:245–252. PMID: 15001303.
58. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991; 115:674–680. PMID: 1929035.
59. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989; 2:1071–1073. PMID: 2572799.
60. Chang FY, Turnidge J. Yu VL, Merigan TC, Barriere S, editors. Staphylococcus aureus. Antimicrobial chemotherapy and vaccines. 1999. 1st ed. Baltimore: Williams & Wilkins;p. 389–404.
61. Mortara LA, Bayer AS. Staphylococcus aureus bacteremia and endocarditis. Infect Dis Clin North Am. 1993; 7:53–68. PMID: 8463653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download