Abstract
Spontaneous splenic rupture (SSR) in a patient undergoing hemodialysis has been described as an extremely rare and potentially fatal complication. We report here spontaneous splenic rupture in a 52-year-old woman undergoing regular hemodialysis for end-stage renal disease (ESRD). She complained of colicky abdominal pain in the left upper quadrant area and dizziness when she assumed an upright posture. Her vital signs revealed low blood pressure and tachycardia, which was suggestive of hypovolemic shock. Abdomen CT scan showed splenic hematoma and hemoperitoneum. However, she had no history of any event triggering the splenic rupture. An exploratory laparotomy showed a ruptured spleen and an emergency splenectomy was performed. We suggest that spontaneous spleen rupture may be attributed to uremic coagulopathy and heparin-induced coagulopathy.
Rupture of the spleen is a potentially life threatening complication usually occurring after blunt trauma to the abdomen. Unlike traumatic splenic rupture, spontaneous non-traumatic splenic rupture is extremely rare. Weidermann first defined the term as resulting from an "incident without external force."1 Knoblich distinguished the non-traumatic rupture of a pathological spleen from the extremely rare case of non-traumatic splenic rupture of an unknown origin.1
Spontaneous splenic rupture in patients undergoing regular hemodialysis is often caused by heparin used in hemodialysis, uremic coagulopathy, infection, secondary amyloidosis, pancreatitis or splenic infarction.2-6 Nevertheless, the etiological diagnosis may be difficult.
We present here a case of spontaneous spleen rupture in a patient undergoing hemodialysis for end-stage renal disease who did not have any recent history of trauma or other precipitating factors of splenic ruptures. The patient was successfully treated with emergency splenectomy.
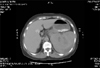
A 52-year-old woman undergoing hemodialysis presented to our hospital complaining of diffuse abdominal distension and colicky abdominal pain on the left upper abdominal quadrant. She had started regular hemodialysis three times (4 hours per time) a week for two years before her arrival at a local hospital. The most recent hemodialysis had been performed the previous day, during which 1,650 units of heparin had been administered as a initial bolus dose followed by an infusion of 500 units of heparin per hour as a maintenance dose. Heparin infusion was maintained for 3 hours without dosage adjustment. She was not taking any anti-hypertensive agents at the time. Erythropoietin was being administered at 6,000 units per week by subcutaneous injection after each hemodialysis session. She had no recent trauma and drug history that would induce bleeding diathesis. She had no abnormal symptoms and signs during hemodialysis. Her vital signs were as follows: body temperature 36.0℃, heart rate 105 beat per min, respiratory rate 20 times per min, and blood pressure 80/60 mmHg. Her conjunctivae were slightly pale, and her abdomen was distended and diffusely tender without rebound or guarding. The remainder of her physical examination was unremarkable. Initial laboratory data revealed hemoglobin 8.0 g/dL, hematocrit 24%, white blood cells 13,980/µL, platelets 259,000/µL, blood urea nitrogen 52 mg/dL, creatinine 6.9 mg/dL, total protein 6.3 g/dL, albumin 3.2 g/dL, total cholesterol 142 mg/dL, total bilirubin 0.6 mg/dL, GOT 49 U/L, GPT 17 U/L, amylase 183 U/L, and lipase 180 U/L. Her prothrombin time (PT) and activated partial thromboplastin time (aPTT) were within normal ranges. Abdomen CT scan showed splenic hematoma and hemoperitoneum (Fig. 1). The patient received 2 units of packed red blood cells during the preparation for the operation. Her systolic blood pressure was elevated to 100 mmHg. An emergency splenectomy was performed without immediate complications related to the surgical procedures. However, 4 units of packed red blood cells were transfused during the operation and dopamine was administered due to low systolic blood pressure (80 mmHg). Because of long-term hypotension, thrombosis was detected within a left arteriovenous fistula (AVF). Therefore, she underwent postoperative hemodiaysis for volume overload and azotemia through a duallumen catheter in the right internal jugular vein. On pathologic examination, the spleen measured 12×11×5 cm, and it weighed 440 g. On microscopic findings, there was subcapsular hematoma with hemorrhage into the underlying splenic parenchyme without any underlying causal pathology (Fig. 2). During her admission, no positive cultures were found in blood, urine and sputum specimens. No evidence of coagulopathy was discovered and clotting agents were not required. By the 15th admission day, the patient was discharged with complete recovery, after her operation for a new AVF.
Spontaneous rupture of the spleen is an extremely rare entity. The known co-morbid conditions are infiltrating malignant cells and extramedullary hematopoiesis,7 spontaneous splenic rupture in splenic infarctions,8 thrombocytopenia,9 amyloidosis,10,11 anticoagulant therapy,12,13 portal hypertension,14 connective tissue disease,15 venous thrombosis in the spleen16 and focal splenic lesions.17
While the diagnosis of splenic rupture may be relatively straightforward in the trauma patient, concurrent disease in an atraumatic patient makes diagnosis challenging. To further complicate matters, splenic rupture has been described with symptoms resembling a wide range of conditions such as cardiovascular disease, cardiogenic shock and scrotal hematomas.18,19
Rapid diagnosis and treatment is required in spontaneous splenic rupture because the condition is potentially fatal. Examination of the abdomen for splenic rupture can be done either with ultrasound or CT scan. The ultrasound signs of splenic rupture are enlargement, displacement, double contour, irregularity of the spleen and intraperitoneal fluid.18 The signs of splenic rupture on the CT scan include foci of hypodensity or hyperdensity that are not enhanced with contrast, and intracapsular, perirenal, and intraperitoneal fluid.20
There is a debate in the literature over whether SSR without a precipitating cause can actually exist.21 While some authors have postulated that this condition results from increased motility or spasm of the splenic vein leading to venous congestion, others believe that the entity simply does not exist.22 However, multiple cases have been reported in which there appeared to be no underlying cause of the SSR.18,22-24
Risk factors associated with SSR are present in uremic hemodialyzed patients, such as the use of anticoagulant (heparin) during hemodialysis, uremic coagulopathy, susceptibility to infectious disease (malaria, infectious mononucleosis, septicemia) due to impaired immunologic functions and amyloidosis, which occurs as a long term complications of hemodialysis.2-6 Therefore, SSR may be more frequent in uremic hemodialyzed patients. However, our patient showed no evidence of with infection, recent trauma, coagulopathy in vitro, or any clinical evidence of malignancy and amyloid deposition into the spleen based on pathologic findings, except for the long-term use of unfractionated heparin during hemodialysis.
All SSR cases do not require splenectomy. Conservative treatment accompanied by ultrasonography can be performed in those who are hemodynamically stable and have no sign of impending rupture. Alternative therapy for spleen preservation are embolization of the splenic artery, subsegmental resection and transposition of the spleen. The advantages of these alternative therapies are spleen mass preservation and prevention of splenectomy-related complications. However, the disadvantages are prolonged hospital stay and delayed operation for subdiaphragmatic collection or delayed spleen rupture.25 The indication for surgical treatment is secondary delayed splenic rupture, increasing amounts of intraabdominal blood, increasing subcapsular hematoma, increasing intraparenchymal bleeding, and non-traumatic intra-splenic pseudoaneurysm.26 The risk of overwhelming postsplenectomy infection (OPSI) varies with age and there is a considerable risk of overwhelming sepsis after splenectomy with a high mortality rate in childhood.27 In our patient, emergency splenectomy was performed immediately owing to the hypovolemic shock during the preoperative and intra-operative state. She was administered pneumococcal vaccine after discharge.
In conclusion, we suggest that the heparin used in hemodialysis and coagulopathy in vivo in uremic patients may be important risk factors related to spleen rupture and subcapsular hematomas. This case also indicates a variety of unique characteristics of spontaneous splenic rupture that should be kept in mind when evaluating patients undergoing hemodialysis who present with abdominal pain and unexplained hypovolemic shock.
Figures and Tables
References
1. Knoblich R. Pathologic (so-called spontaneous) rupture of spleen in leukemia and lymphoma. Mich Med. 1966. 65:105–110.
2. Lund L, Nielsen FB, Sorensen K. Spontaneous rupture of the spleen in a patient with uremia. Clin Nephrol. 1988. 29:107–108.
3. Lloveras JJ, Prevost F, Goudable C, Durand D, That HT, Suc JM. Rupture of the spleen in hemodialyzed patients. Clin Nephrol. 1986. 26:160.
4. Zbrog Z, Pawlicki L. Spontaneous rupture of the spleen as a cause of death of a patient with uremia. Pol Tyg Lek. 1989. 44:232–233.
5. Katsanos KH, Theodorou J, Katopodis KP, Siamoupoulos KC. Spontaneous splenic rupture complicating pancreatitis in a chronic hemodialysis patient. Clin Nephrol. 2004. 61:448–449.
6. Gascon A, Iglesias E, Belvis JJ, Berisa F. The elderly haemodialysis patient with abdominal symptoms and hypovolemic shock-splenic rupture secondary to splenic infarction in a patient with severe atherosclerosis. Nephrol Dial Transplant. 1999. 14:1044–1045.
7. Giagoundis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996. 73:297–302.
8. Yam LT, Crosby WH. Spontaneous rupture of spleen in leukemic reticuloendotheliosis. Am J Surg. 1979. 137:270–273.
9. Levy J. Spontaneous rupture of the spleen in association with idiopathic thrombocytopaenic purpura. Postgrad Med J. 1994. 70:239.
10. Russell TJ, Ferrera PC. Spontaneous rupture of an amyloid spleen in a patient on continuous ambulatory peritoneal dialysis. Am J Emerg Med. 1998. 16:279–280.
11. Dedi R, Bhandari S, Sagar PM, Turney JH. Delayed splenic rupture as a cause of haemoperitoneum in a CAPD patient with amyloidosis. Nephrol Dial Transplant. 2001. 16:2446.
12. Taccone FS, Starc JM, Sculier JP. Splenic spontaneous rupture (SSR) and hemoperitoneum associated with low molecular weight heparin: a case report. Support Care Cancer. 2003. 11:336–338.
13. Weiss SJ, Smith T, Laurin E, Wisner DH. Spontaneous spleen rupture due to subcutaneous heparin therapy. J Emerg Med. 2000. 18:421–426.
14. Thijs JC, Schneider AJ, van Kordelaar JM. Spontaneous rupture of the spleen complicating portal hypertension. Intensive Care Med. 1983. 9:299–300.
15. Tolaymat A, Al-Mousily F, Haafiz AB, Lammert N, Afshari S. Spontaneous rupture of the spleen in a patient with systemic lupus erythematosus. J Rheumatol. 1995. 22:2344–2345.
16. Byerly WG. Thrombosis of the splenic vein with secondary rupture of the spleen. N C Med J. 1975. 36:352–354.
17. Rydell WB Jr, Ellis R. Spontaneous rupture of the spleen from metastatic carcinoma. JAMA. 1978. 240:53–54.
18. Buciuto R, Kald A, Borch K. Spontaneous rupture of the spleen. Eur J Surg. 1992. 158:129–130.
19. Sujka SK, Evans EJ, Nigam A. Delayed rupture of the spleen presenting as a scrotal hematoma. J Trauma. 1986. 26:85–86.
20. Paivansalo M, Myllyla V, Siniluoto T, Kairaluoma MI, Lohela P. Imaging of splenic rupture. Acta Chir Scand. 1986. 152:733–737.
21. Gallerani M, Vanini A, Salmi R, Bertusi M. Spontaneous rupture of the spleen. (letter). Am J Emerg Med. 1996. 14:333–334.
22. Kumar S, Gupta A, Shrivastava UK, Mathur SB. Spontaneous rupture of normal spleen: an enigma recalled. Br J Clin Pract. 1992. 46:67–68.
23. Wergowske GL, Carmody TJ. Splenic rupture from coughing.(letter). Arch Surg. 1983. 118:1127.
24. Lieberman ME, Levitt MA. Spontaneous rupture of the spleen: a case report and literature review. Am J Emerg Med. 1989. 7:28–31.
25. Yaghoobi A. A new technique of splenic preservation: extraperitoneal transposition of the traumatized spleen. Chirurgie. 1997. 122:450–454.
26. Gorg C, Colle J, Gorg K, Prinz H, Zugmaier G. Spontaneous rupture of the spleen: ultrasound patterns, diagnosis and follow-up. Br J Radiol. 2003. 76:704–711.
27. Reihner E, Brismar B. Management of splenic trauma-changing concepts. Eur J Emerg Med. 1995. 2:47–51.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download