Abstract
Breast edema is defined as a mammographic pattern of skin thickening, increased parenchymal density, and interstitial marking. It can be caused by benign or malignant diseases, as a result of a tumor in the dermal lymphatics of the breast, lymphatic congestion caused by breast, lymphatic drainage obstruction, or by congestive heart failure.
Here we describe several conditions, that cause unilateral breast edema with the aim of familiarizing radiologists with these disease entities.
Edema of the breast is characterized by an increased skin thickness and breast parenchymal density with prominent interstitial markings.1 It may co-occur with inflammatory breast carcinoma, lymphatic obstruction (due to axillary, chest wall or intrathoracic lesions), mastitis, fat necrosis, trauma, postirradiation changes, granulomatous diseases, nephrotic syndrome, lymphoma, progressive systemic sclerosis, leukemia, pemphigus and other skin conditions, subclavian or innominate vein occlusion, or congestive heart failure. Thus a knowledge of the etiologies of the entities that cause unilateral breast edema and their typical appearances can aid accurate diagnosis. Here, we describe a spectrum of etiologies and imaging appearances related to unilateral breast edema.
Inflammatory breast carcinoma is an unusual variant of locally advanced breast cancer. Inflammatory breast carcinoma of the breast accounts for 1 - 4% of breast cancers.2 Its clinical manifestations include a redness or purplish discoloration of at least one-third of the breast skin, peau d'orange, and a palpable ridge at the margin of induration. Mass, tenderness or pain, increased temperature of the involved breast, and breast enlargement are often present. Pathologically, any subtype of primary breast carcinoma may be present, but dermal lymphatic vessels must be involved3 and subepidermal capillaries and venules may also be occluded. Tumor involvement and obstruction of lymphatic vessels and capillaries result in mammographic findings of stromal coarsening, and the thickening of Cooper ligaments and the skin.4
The mammographic findings of inflammatory breast carcinoma are a mass, malignant-type calcifications, and skin changes4 (Fig. 1). Moreover, because inflammatory breast carcinoma has the nature of an advanced cancer, axillary lymphadenpathy is commonly present. Sometimes breast infection has similar mammographic findings, but without malignant microcalcifications. And thus, a careful evaluation of imaging findings and clinical history is needed, because the detection of malignant microcalcifications by mammography and axillary lymphadenopathy can be underestimated.5
Paulus et al. reported that metastasis to the breast may occur by two distinct routes, i.e., lymphatic or blood borne.6 Of these, lymphangitic metastasis to the breast usually occurs via transthoracic or cross-lymphatic metastasis from contralateral primary breast cancer.
Mammographic findings of lymphangitic metastasis are skin thickening, denser subcutaneous tissue with a thicker trabecular pattern, and denser and more irregular glandular stroma.6 Lymphangitic metastasis of the breast has clinical symptoms that are similar to inflammatory breast cancer, however, radiographically detected by mammography and US microcalcifications or masses are more frequent in lymphangitic metastasis than in inflammatory breast cancer. Thus cases of lymphangitic metastasis with mass formation7 are difficult to differentiate from inflammatory breast carcinoma.
The breast is a relatively uncommon hematogenous metastasis site from an extramammary malignancy. Melanoma and lymphomas are the most common sources of metastasis. According to a recent report,7 stomach cancer is the second most common cause of breast metastasis in South Korea, possibly because of the high incidence of stomach cancer. The most common radiographic appearance of metastasis is the presence of one or more round, discrete nodules without microcalcifications, although metastatic ovarian carcinoma is an exception in this report. In addition some have reported breast metastasis mimicking inflammatory breast carcinoma (Fig. 2).
Malignant lymphoma can originate as a primary breast tumor, or it may involve the breast secondarily as part of a diffuse metastatic process. Primary malignant lymphomas of the breast comprise 0.05% to 0.53% of malignant breast tumors.10 Most reported cases of primary breast lymphoma have been of the non-Hodgkin's variety. Secondary involvement in patients with a history of malignant lymphoma is somewhat more common. Clinically breast lymphoma can occasionally present as a diffuse rapid breast enlargement in younger age groups, or as breast skin thickening due to lymphatic blockage by lymphoma resulting in retrograde edema.11
The mammographic findings of lymphoma are a solitary noncalcified mass, multiple masses, diffuse increased opacity with skin thickening (Fig. 3), and negative descriptive findings. Breast lymphomas lack the calcification and spiculated margin, features characteristic of breast carcinoma. By US they are often hypoechoic and may be almost anechoic.12 Because of their nonspecific appearance by radiographic examination, Paulus suggested that the only significant clue concerning the presence of lymphomatous disease in the breast, is the presence of bilateral axillary lymph node enlargement.6
The diagnosis of primary lymphoma of the breast requires pathologic evidence of the close apposition of a lymphomatous infiltrate and normal breast tissue in patients with neither a previous nor a concurrent lymphoma at another site. Ipsilateral axillary nodes are acceptable, provided that these lesions develop in parallel with tumors. Axillary node involvement in patients with lymphoma of the breast has been reported to occur in 30 - 40%.13 The prognosis of lymphoma of the breast appears to be similar to that of nodal lymphoma, given an equivalent stage and histology.14
Most commonly, breast infection occurs in young women, especially in the lactating state. But sometimes, breast infection occurs in a reduced immune state, such as, in those with diabetes mellitus, ductectasia, or galactocele. Because the breast is filled with milk, it tends to be susceptible to bacterial infections. Common organisms are staphylococcus and streptococcus, though tuberculosis may sometimes be encountered.
The most common mammographic finding is an irregular mass, whereas diffuse breast edema is observed in only a minority of patients15 (Fig. 4). These findings overlap with those of inflammatory breast carcinoma. But, diffuse skin thickening and dense lymph nodes favor inflammatory breast carcinoma,15 and are rare in cases of breast infection, except when the breast infection is unusual.
Tuberculosis of the breast has variable findings, including a coarse parenchymal texture, skin thickening, nipple retraction, the absence of microcalcifications, and a cloudy breast density with a sinus tract and skin bulge.16 Moreover, later the affected breast becomes smaller that the unaffected breast (Fig. 5). Three forms of tuberculosis have been reported, namely, the nodular, disseminated and sclerosing forms.17 In the disseminated form, mammography shows diffuse edema. Tuberculosis of the breast is difficult to differentiate from advanced breast carcinoma. However, in cases of malignancy, microcalcifications, nodular or spiculated masses, and axillary lymphadenopathy are more common. Furthermore, in malignancy, the affected breast is often larger, which contrasts with the situation in tuberculosis of the breast.
If inflammatory signs are not improved by treatment, a biopsy is indicated to eliminate inflammatory breast carcinoma. Ultrasound may be a useful adjunct by delineating a hypoechoic irregularly shaped nodule or by demonstrating focal abnormal acoustic shadowing.
Unilateral breast edema may be due to a tendency to lie on one side, causing dependent edema18 (Fig. 6), and the atrophic breasts of elderly women may be more susceptible. If changes are unilateral, the possibility of carcinoma is of particular concern since systemic diseases usually give rise to bilateral abnormalities. Pitting edema of the breast and the absence of a palpable breast mass are helpful for distinguishing between breast edema due to congestive heart failure and malignancy,19 and after treatment for heart failure the breast edema should resolve.
Postoperative edema has been reported to occur in 41%, and to be related to the axillary staging procedure.20 The usual mammographic findings are skin thickening, coarsening of stroma, an increased breast density (Fig. 7), and later benign calcifications.21 Responses of tissue to lumpectomy and radiation, such as, breast edema and skin thickening, are most pronounced 6 to 12 months after treatment, and gradually resolve within 1 to 3 years.22 Histologically, architectural distortion, fibrosis, and atrophy develop in treated breasts to various extents. Moreover, the same breast may show several types of change, which are not necessarily related to the radiation dose. Although the definite pathophysiologies of postsurgical changes are not known, it has been suggested that they are caused by the interruption of lymphatic vessels and associated lymphostasis.
To differentiate post-operative edema from other causes of unilateral breast edema, knowledge of clinical history is important. However, when sequential mammograms reveal persistent or increased breast edema recurrent carcinoma should be considered.
Mechanical problems such as obstruction due to lymph node enlargement, subclavian vein occlusion, and especially arteriovenous hemodialysis complications, may cause unilateral breast edema.23 In such cases, patient history and enlarged lymph nodes by ultrasonography are useful guidelines for differentiating them from other diseases which cause unilateral breast edema.
Figures and Tables
Fig. 1
Inflammatory breast cancer in a 40-year-old woman with a palpable mass and erythematous change of the right breast. Mediolateral mammograms show diffuse increased density, trabecular thickening, skin thickening, and a spiculated mass on the right upper portion (arrows).
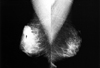
Fig. 2
Metastasis in a 43-year-old woman with left breast swelling and skin change. The patient had a signet ring cell carcinoma on her stomach. Biopsy specimens of the hypoechoic nodule in the left breast were evaluated microscopically and immunohistochemically, which included estrogen receptor (ER) and gross cystic disease fluid protein-15 (GCDFP-15), for differentiating primary and metastatic breast cancer. However, the immunohistochemical results for ER and GCDFP-15 were negative, indicating a metastatic signet ring cell carcinoma of the breast. A & B, Mediolateral and craniocaudal mammograms show diffuse increased density, trabecular thickening, and skin change without a definite focal mass. C, Sonogram shows diffuse skin and subcutaneous edema and two suspicious hypoechoic nodules in the left upper outer.
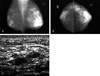
Fig. 3
Breast lymphoma in a 35-year-old woman with a right breast enlargement. Mediolateral oblique mammogram shows diffuse increased opacity throughout the entire right breast with skin thickening.
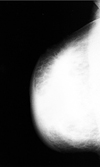
Fig. 4
Mastitis in a 37-year-old woman with breast pain, swelling, and an erythematous change of right breast of 2 weeks duration. After antibiotic treatment the clinical symptoms disappeared. A & B, Mediolateral and craniocaudal mammogram show diffuse skin thickening and accentuated Cooper's ligament.
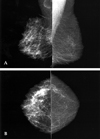
Fig. 5
Tuberculous abscess in an 81-year-old woman with a painful swelling of the left breast of 2 months duration. A & B, Mediolateral and craniocaudal mammogram show skin thickening, trabecular thickening, and diffuse increased density.
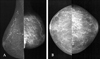
Fig. 6
Congestive heart failure in a 47-year-old woman who complained of dyspnea, dizziness, and a swelling of the left breast. A, Initial craniocaudal mammogram shows diffuse increased density with reticular fascial thickening and marked skin thickening. B, Follow-up left craniocaudal mammogram after 4 weeks of diuretic therapy showing significant resolution of the increased density and skin thickening. C, Sonogram shows marked skin thickening, and an echogenic subcutaneous fat layer with tubular and reticular anechoic structures, suggestive of dilated lymphatics (left), as compared with the normal side (right).
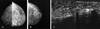
Fig. 7
Post-radiotherapeutic change in a 71-year-old woman due to a poorly differentiated carcinoma of the breast. (A & B) Mediolateral and craniocaudal mammogram show a spiculated mass in upper outer portion of right breast. (C) After partial mastectomy and radiotherapy, this craniocaudal mammography showed diffuse increased density and trabecular and skin thickening as compared to the later 2-year follow-up mammography (D).

References
1. Kim EK, Lee SK, Oh KK. Mammographic and sonographic findings of unilateral breast edema in congestive heart failure: a case report. J Korean Radiol Soc. 1997. 36:1097–1099.
2. Swain SM, Lippman M. Bland KI, Copeland EM, editors. Locally advanced breast cancer. The breast: comprehensive management of benign and malignant diseases. 1991. Philadelphia, PA: Saunders;843–862.
3. Ellis DL, Teitelbaum SL. Inflammatory carcinoma of the breast: a pathologic definition. Cancer. 1974. 33:1045–1047.
4. Dershaw DD, Moore MP, Liberman L, Deutch BM. Inflammatory breast carcinoma: mammographic findings. Radiology. 1994. 190:831–834.
5. Tardivon AA, Viala J, Corvellec Rudelli A, Guinebretiere JM, Vanel D. Mammographic patterns of inflammatory breast carcinoma: a retrospective study of 92 cases. Eur J Radiol. 1997. 24:124–130.
6. Paulus DD, Libshitz HI. Metastasis to the breast. Radiol Clin North Am. 1982. 20:561–568.
7. Chung SY, Oh KK. Imaging findings of metastatic disease to the breast. Yonsei Med J. 2001. 42:497–502.
8. Hajdu SL, Urban JA. Cancers metastatic to the breast. Cancer. 1972. 29:1691–1696.
9. Vizcaino I, Torregrosa A, Higueras V, Morote V, Cremades A, Torres V, et al. Metastasis to the breast from extramammary malignancies : a report of four cases and a review of literature. Eur Radiol. 2001. 11:1659–1665.
10. Petrek JA. Harris JR, Hellman S, Henderson IC, Kinne DW, editors. Lymphoma. Breast diseases. 1991. 2nd. Philadelphia, Pa: Lippincott;806–807.
11. Watson AP, Fraser SE. Primary lymphoma of the breast. Australas Radiol. 2000. 44:234–236.
12. Liberman L, Giess CS, Dershaw DD, Louie DC, Deutch BM. Non-Hodgkin lymphoma of the breast: imaging characteristics and correlation with histopathologic findings. Radiology. 1994. 192:157–160.
13. Schouten JT, Weese JL, Carbone PP. Lymphoma of the breast. Ann Surg. 1981. 194:749–753.
14. Dao AH, Adkins RB Jr, Glick AD. Malignant lymphoma of the breast: a review of 13 cases. Am Surg. 1992. 58:792–796.
15. Crowe DJ, Helvie MA, Wilson TE. Breast Infection. Mammographic and sonographic findings with clinical correlation. Invest Radiol. 1995. 30:582–587.
16. Makanjuola D, Murshid K, Al Sulaimani S, Al Saleh M. Mammographic features of breast tuberculosis: the skin bulge and sinus tract sign. Clin Radiol. 1996. 51:354–358.
17. Tabar L, Kett K, Nemeth N. Tuberculosis of the breast. Radiology. 1976. 118:587–589.
18. Doyle AJ. Unilateral breast edema in congestive heart failure-a mimic of diffuse carcinoma. Australas Radiol. 1991. 35:274–275.
19. Duncan G, Smith R. Cardiac failure mimicking advanced breast carcinoma. Practitioner. 1989. 223:1436–1439.
20. Clarke D, Martinez A, Cox RS, Goffinet DR. Breast edema following staging axillary node dissection in patients with breast carcinoma treated by radical radiotherapy. Cancer. 1982. 49:2295–2299.
21. Dershaw DD, Shank B, Reisinger S. Mammographic findings after breast cancer treatment with local excision and definitive irradiation. Radiology. 1987. 164:455–461.
22. Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am. 1992. 30:107–138.
23. Gadallah MF, el-Shahawy MA, Campese VM. Unilateral breast enlargement secondary to hemodialysis arteriovenous fistula and subclavian vein occlusion. Nephron. 1993. 63:351–353.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download