Abstract
Objective
To assess the efficacy and safety of thyroid radiofrequency (RF) ablation for benign thyroid nodules by trained radiologists according to a unified protocol in a multi-center study.
Materials and Methods
From 2010 to 2011, 345 nodules from 345 patients (M:F = 43:302; mean age ± SD = 46.0 ± 12.7 years, range = 15–79) who met eligibility criteria were enrolled from five institutions. At pre-ablation, the mean volume was 14.2 ± 13.2 mL (1.1–80.8 mL). For 12 months or longer after treatment, 276 lesions, consisting of 248 solid and 28 predominantly cystic nodules, were followed. All operators performed RF ablation with a cool-tip RF system and two standard techniques (a transisthmic approach and the moving-shot technique). Volume reduction at 12 months after RF ablation (the primary outcome), therapeutic success, improvement of symptoms as well as of cosmetic problems, and complications were evaluated. Multiple linear regression analysis was applied to identify factors that were independently predictive of volume reduction.
Results
The mean volume reduction at 12 months was 80.3% (n = 276) and at the 24-, 36-, 48-, and 60-month follow-ups 84.3% (n = 198), 89.2% (n = 128), 91.9% (n = 57), and 95.3% (n = 6), respectively. Our therapeutic success was 97.8%. Both mean symptom and cosmetic scores showed significant improvements (p < 0.001). The rate of major complications was 1.0% (3/276). Solidity and applied energy were independent factors that predicted volume reduction.
For patients with benign nonfunctioning thyroid nodules, current recommendations for radiofrequency (RF) ablation in Korea and Italy indicate treatment when patients complain of nodule-related compressive symptoms or cosmetic deformity (12). For treatment, surgery is the traditional method. However, nonsurgical treatments, such as ethanol, laser, and RF ablation, have been used in recent years with good clinical outcomes (34). RF ablation guided by ultrasound (US) has achieved robust improvement of nodule-related symptoms and cosmetic problems by reducing the nodule volume. Indeed, the volume reduction ratio has been reported to be 84.1% and 79.4% by Korean (5) and Italian groups (6), respectively. In subsequent studies, wide ranges of volume reduction (51–92%) achieved by RF ablation have been published (3789). This variation may be a consequence of several factors, such as characteristics of the treated nodules, the number of treatment sessions, devices used, and operator experience (1101112). Although the efficacy of RF ablation for thyroid nodules has been reported by many centers (6791013141516), most investigations have been either retrospective or single-center studies which have limitations regarding generalizability. Therefore, the generalizability of RF ablation and its acceptable efficacy have remained uncertain.
The Korean Society of Thyroid Radiology (KSThR) has established an annual training system for thyroid RF ablation and publishes retrospective thyroid RF studies by thyroid radiologists who train in this program (1718). To the best of our knowledge, no prospective multicenter study has demonstrated the generalizability of thyroid RF ablation by trained radiologists. Therefore, the KSThR designed this prospective multicenter study to prove the efficacy and safety of thyroid RF ablation for nonfunctioning thyroid nodules by trained radiologists who followed a unified protocol and used similar devices.
This prospective multicenter study was approved by the Institutional Review Boards of the five participating centers (Asan Medical Center [2009-0727], Seoul St. Mary's Hospital [KC1ORIME0404], Daerim St. Mary's Hospital [200903], Seoul National University Hospital [0912-062-304], Human Medical Imaging and Intervention Center [HI201002]).
This study was designed by the research committee of the KSThR, which, in November 2009, sent an e-mail to KSThR members at 13 hospitals who had undergone a RF ablation training course and had experience with > 50 cases of thyroid RF ablation (17). We defined a “trained radiologist” as one who successfully completed more than 50 cases of thyroid RF ablation and had participated in the RF ablation programs organized by the KSThR. A total of five hospitals applied to participate in this study and seven radiologists at these institutions had completed RF sessions of more than 50 cases and with 3–10 years of experience. From May 2010 to December 2011, 345 nodules from 345 patients (M:F = 43:302, mean age ± SD = 46.0 ± 12.7 years, range = 15–79) who met the eligibility criteria and provided written informed consent were enrolled.
The inclusion criteria were as follows: patients with symptomatic problems due to a thyroid nodule; patients with cosmetic thyroid problems; cytological confirmation of a benign thyroid nodule on two separate US-guided biopsies (119); no malignant US findings (19); solid (> 50% solid components) and predominantly cystic (10% < solid components < 50%) thyroid nodules; and serum thyroid hormone and thyrotropin levels within normal ranges. The exclusion criteria were (1) follicular neoplasm or primary thyroid cancer; history of neck radiation therapy; pregnancy; and cystic nodules (< 10% solid components). Ethanol ablation was suggested as the first-line treatment for cystic nodules (< 10% solids) (20).
All patients were evaluated by US examination, US-guided biopsy, blood tests, and clinical examinations. US, US-guided biopsy, and RF ablation were performed using a 10–16 MHz linear probe and a real-time US system (EUB-7500, Hitachi Medical Systems, Tokyo, Japan; iU22 and HDI-5000, Philips Healthcare, Bothell, WA, USA; Aplio SSA-770A, Toshiba Medical Systems Corporation, Otawara-shi, Japan; and Accuvix XG, Samsung Medison, Seoul, Korea). For each nodule, three orthogonal diameters (the largest diameter and two perpendicular diameters) and the proportion of the solid components were measured (5). The volume of each nodule was calculated as follows: V = πabc/6 (where V is the volume, a is the largest diameter, and b and c are the two perpendicular diameters) (5). Vascularity was graded using a four-point scale (grade 0, no vascularity; grade 1, perinodular vascularity only; grade 2, intranodular vascularity < 50%; and grade 3, intranodular vascularity > 50%). Laboratory examinations included measurements of serum thyrotropin, total triiodothyronine, free thyroxine, platelet counts, and blood coagulation tests that included prothrombin time and activated partial thromboplastin time (1). At the time of enrollment, the patients were asked to rate their nodule-related symptoms on a 10-cm visual analog scale (0–10 cm) (1) and a cosmetic grading score was assessed by the physician (1, no palpable mass; 2, no cosmetic problem but a palpable mass; 3, a cosmetic problem on swallowing only; and 4, an easily detected cosmetic problem) (1).
All procedures were performed under US guidance on an outpatient basis by seven radiologists. The RF device used was a cool-tip RF system with a straight-type modified internally cooled electrode: RF generators (Cool-Tip RF system, Covidien, Boulder, CO, USA; SSP-2000, Taewoong Medical, Gimpo, Korea; and M-1004, RF Medical, Seoul, Korea) and an 18-gauge internally cooled electrode (Well-Point RF Electrodes, Taewoong Medical, Gyeonggi-do, Korea; VIVA, STARmed, Gyeonggi-do, Korea; Big-Tip, RF Medical, Seoul, Korea; Cool-tip RF system, Radionics, Valleylab, Colo., USA) with 0.7-, 1-, 1.5-, and 2-cm active tips, respectively, depending on the size of the nodule and the preference of the radiologists. The needle sizes (with respective nodule sizes) were as follows: 0.7 cm (< 3 cm), 1.0 cm (< 4 cm), and 1.5 cm or 2 cm (> 4 cm).
A transisthmic approach method and the moving-shot technique were used (121). To prevent hemorrhage, vessels along the approach route were carefully evaluated using Doppler US. Patients were managed with 1–2% lidocaine around the thyroid gland for puncture site anesthesia. A transisthmic approach allows the electrode to pass through a sufficient amount of thyroid parenchyma by insertion of the electrode through the short axis of the target nodule from the isthmus. This method has several advantages as it can prevent movement of the needle or electrode when the patient swallows or talks during ablation and it can also prevent fluid leakage (i.e., ablated hot fluid of the thyroid nodules' cystic portion) outside the thyroid gland. Moreover, this approach also allows continuous US monitoring of the nodule as well as of the space between the electrode tip and the expected location of the recurrent laryngeal nerve, thereby minimizing the risk of injury to the nerve and/or the esophagus. An electrode was inserted into the thyroid nodule under US guidance along the thyroid nodule. Initially, the electrode tip was positioned in the deepest and most remote portion of the nodule. Ablation was initiated with RF power ranging from 15 W to 80 W according to the electrode tip size: 0.7 cm, 15–30 W; 1 cm, 30–70 W; and 1.5 cm and 2 cm, 80 W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 seconds, the RF power was increased in 5–10 W increments, up to 120 W according to the electrode as follows: 0.7 cm, 40–70 W; 1 cm, 55–90 W; and 1.5 cm and 2 cm, 100–120 W. When a transient hyperechoic zone appeared at the periphery of the nodule (usually within 5–10 seconds), the electrode tip was moved backwards for the prevention of heat transmission to the perithyroidal tissue. In the nodule's central areas, the electrode was moved to an untreated area if the transient hyperechoic zone expanded around the electrode tip. This technique was termed the “moving-shot technique,” in contrast to the “fixed-needle technique” that is normally used to treat hepatocellular carcinoma. Before ablation, the nodule was divided into multiple predicted ablation units. These units were designed to be smaller in the periphery of the nodule and its portion adjacent to critical structures of the neck, but much larger in the nodule's central portion. Next, the nodule was treated “unit by unit” using the moving-shot technique. If the patient could not tolerate pain during the ablation, the power was either turned off for several minutes or lidocaine was injected around the thyroid capsule. The ablation procedure was terminated when the entire nodule had become hyperechoic. Potential complications were evaluated according to clinical signs and symptoms both during and immediately after the procedure. Complications and side effects were defined according to the quality improvement guidelines of the Society of Interventional Radiology (22) and a prior multicenter evaluation of complications (17). After RF ablation, each patient was observed in the hospital for 1–6 hours.
Patients were followed up by US and clinical evaluations at one and 12 months, and then annually up to five years. The thyroid nodule volume, largest diameter, vascularity, and cosmetic as well as symptom scores were evaluated in the same manner before and after ablation. The volume reduction ratio was calculated as follows: volume reduction ratio = ([initial volume - final volume] × 100) / initial volume (10). Therapeutic success was defined as a > 50% volume reduction at 12 months. Additional treatment was allowed if the follow-up US showed a remaining viable portion of the nodule and if the patient complained of incompletely (< 50%) resolved symptomatic or cosmetic problems. All complications were also evaluated during the follow-up. A delayed complication was defined as any complication that was detected at one month or more after RF ablation (17).
The primary outcome was the volume reduction at 12 months after the RF ablation. Outcomes such as changes in the largest diameter, volume, and symptom score, or the cosmetic score before and after RF ablation were compared employing the Wilcoxon signed-rank test. Data were analyzed using IBM SPSS, version 21 (IBM Corp., Armonk, NY, USA). Continuous variables are reported as the means ± SD with the range. A multiple linear regression analysis was calculated to identify factors that were independently predictive of efficacy (i.e., the volume reduction ratio at 12 months). Variables entered into the model included age, gender, number of treatment sessions, mean energy per mL pretreatment nodule volume, initial nodule volume, initial nodule solidity, and initial nodule vascularity. A p value less than 0.05 was considered statistically significant.
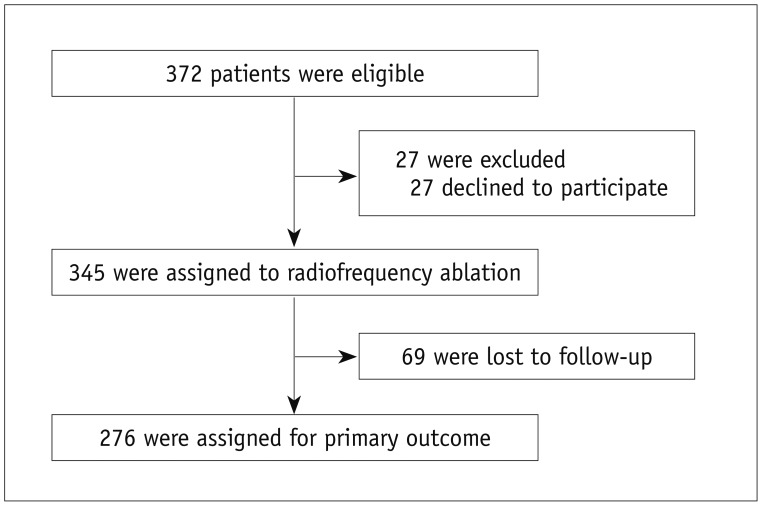
From May 2010 to December 2011, 372 nodules from 372 patients (Fig. 1) with a benign thyroid mass were identified by attending physicians at the five participating centers. After excluding 27 patients who had declined to participate, 345 patients were assigned to RF ablation. Following treatment, 69 patients were lost before the 12-month follow-up. A final sample of 276 patients who were followed for 12 months or longer (M:F = 32:244, mean age ± SD = 46.3 ± 12.8 years, range = 15–79) was included in the analyses. The number of included nodules per institution ranged from 3 to 123 (3, 107, 123, 13, and 30 nodules, respectively).
Demographic characteristics of all enrolled patients (n = 345) are summarized in Table 1. Before RF ablation, the mean largest diameter of the thyroid nodules was 3.8 ± 1.1 (range 1.9–8.0) cm with a mean volume of 14.2 ± 13.2 (range 1.1–80.8) mL. The mean degree of vascularity was grade 2.0 ± 0.8 (range 0–3) and blood tests were within normal limits in all patients. Among the 276 treated thyroid nodules, there were 248 solid and 28 predominantly cystic nodules. A 1-cm active tip was used in 79.7% (220/276) of the patients and electrodes of other sizes in the other subjects (0.7 cm in 13 patients, 1.5 cm in 40 patients, and 2 cm in three patients). The mean volume for the single-session group was significantly smaller than that of the group with two treatment sessions (10.7 mL vs. 23.0 mL, p < 0.001). All patients tolerated RF ablation and none were transferred to surgery during the follow-up.
For the primary outcome of nodule volume reduction, the absolute volume reduction was 80.3 ± 13.7% (range 38.7–100%) at the 12-month follow-up and the individual rates from each institution were as follows: 81.8, 77.2, 83.4, 80.4, and 78.7%.
Treatment characteristics of our study patients are summarized in Table 2. The mean total energy delivered per mL pre-treatment nodule volume was 4161.5 ± 2992.8 (range 656.3–22030.6) J. The mean time to additional RF ablation was 8.5 ± 7.5 (range 1–36) months after the initial RF ablation. The mean number of performed RF sessions was 1.3 ± 0.4 (range 1–2; one session in 206 patients and two in 70). The treatment outcomes are summarized in Table 3. The mean volume reductions at the 1-, 6-, 12-, 24-, 36-, 48-, and 60-month follow-ups were 44.4 ± 17.0% (n = 276, range 3.1–86.0%), 69.1 ± 17.0% (n = 276, range 8.1–98.3%), 80.3 ± 13.7% (n = 276, range 38.7–100%), 84.3 ± 13.2% (n = 198, range 36.1–100%), 89.2 ± 10.8% (n = 128, range 45.5–100%), 91.9 ± 8.4% (n = 57, range 69.9–100%), and 95.3 ± 4.3% (n = 6, range 88.5–100%), respectively. The therapeutic success rate was 97.8% (270/276) at the 12-month follow-up. The individual success rates at each institution were as follows: 100, 97, 98, 100, and 93%. Results of the multiple linear regression analysis are summarized in Table 4, which revealed that solidity (p < 0.001) and energy delivered (p = 0.01) were independent factors that predicted the final volume reduction.
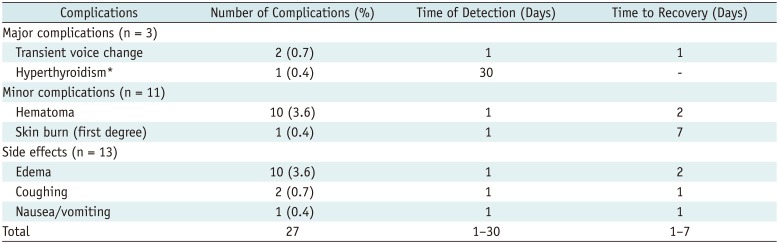
Complications and side effects are summarized in Table 5. The overall complication rate was 5.1% (14/276). Major and minor complication rates were 1.1% (3/276) and 4.0% (11/276), respectively. The side-effect rate was 4.7% (13/276). Almost all study subjects recovered without sequelae; only one hyperthyroid patient was treated with medication (propylthiouracil, 100–150 mg/day). Therefore, the overall sequelae rate was 0.36% (1/276). No patient experienced a life-threatening or delayed complication during the follow-up.
Our current prospective multicenter study was performed by trained radiologists using similar techniques and devices, which demonstrated the efficacy and safety of RF ablation. The primary outcome of mean volume reduction in the present study was 80.3% at 12 months, which is comparable to that reported in a large population study by Jeong et al. (5) (84.1% at 12-month follow-up). Additionally, our investigation achieved a 95.3% volume reduction at the five-year follow-up. The major complication rate in our study was 1.1%, which is comparable to that of a previous multicenter study with a large sample (1.4%, n = 1459) (17). Moreover, there were no life-threatening complications. Solidity and applied energy were found to be independent factors that predicted the final volume reduction.
The value of our present findings lies in validating the generalizability of RF ablation for nonfunctioning benign thyroid nodules in a prospective large population multicenter study. Additionally, trained operators, unified treatment protocols, and similar devices were used in our current investigation. Although the efficacy of thyroid RF ablation has been suggested in many previous reports, such as retrospective studies (56), randomized controlled trials (912142324), review articles (42526), well as one systematic review and one meta-analysis each (327), the generalizability of RF ablation has not been well-established. The KSThR has been organizing a thyroid RF training program since 2007 (17). For our study, we invited radiologists who had undertaken that training program and had experience with more than 50 cases of thyroid RF ablation. A previous study (17) revealed that patients treated by experienced radiologists showed fewer complications than less experienced radiologists (< 50 cases). To date, there is no report on the learning curve for the operators in RF ablation of thyroid lesions; however, some studies suggest that experiences with more than 50 cases could represent the cut-off number to indicate a high success rate for the procedure. Our study also used comparable devices, namely cool-tip RF systems with thyroid-dedicated electrodes (straight-type modified internally cooled electrodes) (28). Additionally, we employed a similar method that involved a transisthmic approach and the moving-shot technique (21). Overall, the trained operators could achieve effective and safe results.
Regarding efficacy, the volume reduction achieved in the present study is comparable to those of previous studies from Korea (5) and Italy (6) with 236 and 94 patients, respectively. Although the initial volume in our investigation (14.2 mL) was larger than that of a previous study (6.1 mL) (5), the primary outcome showed equivalent results. When we compared our findings with those of a laser ablation study (29), the volume reduction was similar or even slightly superior (59% vs. 80.3% at the one-year follow-up).
With respect to safety, our research revealed a complication rate that was comparable with that of a prior large population study for major complications (1.4% vs. 1.0%) and sequelae (0.14% vs. 0.3%) (17). Additionally, no life-threatening complications were observed in our findings. These low complication rates suggest that operators who have experience with more than 50 cases can perform safe RF ablation procedures. Moreover, all of our patients with complications spontaneously recovered within seven days, although one subject developed permanent hyperthyroidism that necessitated additional treatment with medication. Transient hyperthyroidism has also been reported in prior investigations (530) with spontaneous recovery of the affected patients within one month. Therefore, a patient complaining of hyperthyroid symptoms after RF ablation should be carefully evaluated.
Previous RF studies reported a wide range of volume reduction from 50.5% to 93.5% (5678910121520232830313233) and, notably, four of them > 90% (8102031). This excellent volume reduction was induced by the enrollment of cystic nodules (in four studies), the combined use of ethanol and RF ablation (two studies), and a longer (four-year) follow-up period (one study). In our present investigation, cystic nodules were excluded and no patient received RF combined with ethanol. Although the efficacy and safety of this procedure have been reported previously, assessing the involvement of different participants (operators) and geographic locations (institutions), along with a wider range of populations (nodule characteristics) will be necessary to validate the general applicability of thyroid RF ablation. Our current study involved trained operators from different hospitals, 276 nodules with different characteristics (volume, solidity, and vascularity), and a regular follow-up period after RF ablation (one and 12 months).
Regarding the factors related to volume reduction, earlier RF research suggested that variables such as the initial nodule volume (1012), proportion of solid components (581031), and follow-up period (510) are related to volume reduction. In contrast, our analyses found that initial solidity and applied energy were independent predictors of nodule volume reduction. Lim et al. (10) previously suggested that a solid nodule or larger initial volume require more energy for sufficient volume reduction. Recently, Ha et al. (26) reported that the proportion of solid components is important for the treatment modality selection.
Our study has several limitations. First, only Korean radiologists participated. An international prospective multicenter study with radiologists trained in an international program will be necessary to validate RF ablation as a universally applicable procedure. Second, the follow-up period was limited to 12 months and our shortterm follow-up data could not evaluate the long-term recurrence of treated nodules. However, a previous RF study showed that volume reduction is usually minimal after 12 months and that the recurrence rate (5.6%) is low (10). Lim et al. (10) reported mean volume reduction rates of 89.9% and 93.5% at the one- and four-year follow-up, respectively. Those authors reported that only a 3.6% volume reduction was achieved in the two- and four-year follow-up period.
In conclusion, RF ablation performed by trained radiologists from multiple institutions using a unified protocol and similar devices was effective and safe for treating benign thyroid nodules.
References
1. Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13:117–125. PMID: 22438678.

2. Garberoglio R, Aliberti C, Appetecchia M, Attard M, Boccuzzi G, Boraso F, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. 2015; 18:423–430. PMID: 26550079.

3. Fuller CW, Nguyen SA, Lohia S, Gillespie MB. Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. Laryngoscope. 2014; 124:346–353. PMID: 24122763.

4. Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013; 98:3949–3957. PMID: 23956350.
5. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008; 18:1244–1250. PMID: 18286289.

6. Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid. 2009; 19:219–225. PMID: 19265492.

7. Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008; 34:784–791. PMID: 18207307.

8. Lee JH, Kim YS, Lee D, Choi H, Yoo H, Baek JH. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg. 2010; 34:1488–1493. PMID: 20376445.

9. Faggiano A, Ramundo V, Assanti AP, Fonderico F, Macchia PE, Misso C, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. J Clin Endocrinol Metab. 2012; 97:4439–4445. PMID: 23019349.

10. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013; 23:1044–1049. PMID: 23096937.

11. Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011; 12:525–540. PMID: 21927553.

12. Huh JY, Baek JH, Choi H, Kim JK, Lee JH. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session--prospective randomized study. Radiology. 2012; 263:909–916. PMID: 22438360.

13. Deandrea M, Sung JY, Limone P, Mormile A, Garino F, Ragazzoni F, et al. Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid. 2015; 25:890–896. PMID: 26061686.

14. Cesareo R, Pasqualini V, Simeoni C, Sacchi M, Saralli E, Campagna G, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015; 100:460–466. PMID: 25387256.

15. Turtulici G, Orlandi D, Corazza A, Sartoris R, Derchi LE, Silvestri E, et al. Percutaneous radiofrequency ablation of benign thyroid nodules assisted by a virtual needle tracking system. Ultrasound Med Biol. 2014; 40:1447–1452. PMID: 24785443.

16. Ugurlu MU, Uprak K, Akpinar IN, Attaallah W, Yegen C, Gulluoglu BM. Radiofrequency ablation of benign symptomatic thyroid nodules: prospective safety and efficacy study. World J Surg. 2015; 39:961–968. PMID: 25446486.

17. Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012; 262:335–342. PMID: 21998044.

18. Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015; 25:112–117. PMID: 25320840.

19. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH. Korean Society of Thyroid Radiology (KSThR). Korean Society of Radiology. Ultrasound-guided fine needle aspiration of thyroid nodules: A Consensus Statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015; 16:391–401. PMID: 25741201.

20. Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013; 269:293–300. PMID: 23616630.

21. Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014; 15:836–843. PMID: 25469097.
22. Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S243–S246. PMID: 14514826.

23. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010; 194:1137–1142. PMID: 20308523.

24. Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015; 16:1332–1340. PMID: 26576124.

25. Wong KP, Lang BH. Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol. 2013; 2013:428363. PMID: 24298282.

26. Ha EJ, Baek JH. Advances in nonsurgical treatment of benign thyroid nodules. Future Oncol. 2014; 10:1399–1405. PMID: 25052750.

27. Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015; 100:1903–1911. PMID: 25695887.

28. Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg. 2009; 33:1971–1977. PMID: 19575141.

29. Papini E, Rago T, Gambelunghe G, Valcavi R, Bizzarri G, Vitti P, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014; 99:3653–3659. PMID: 25050903.

30. Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006; 16:361–367. PMID: 16646682.

31. Jang SW, Baek JH, Kim JK, Sung JY, Choi H, Lim HK, et al. How to manage the patients with unsatisfactory results after ethanol ablation for thyroid nodules: role of radiofrequency ablation. Eur J Radiol. 2012; 81:905–910. PMID: 21388767.

32. Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006; 65:364–368. PMID: 16918957.

33. Che Y, Jin S, Shi C, Wang L, Zhang X, Li Y, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015; 36:1321–1325. PMID: 25814656.

Table 1
Demographic Characteristics of Enrolled Patients
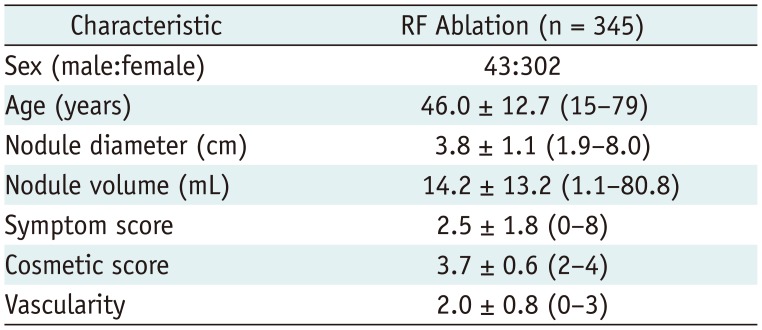
Table 2
Treatment Characteristics of 276 Thyroid Nodules Analyzed
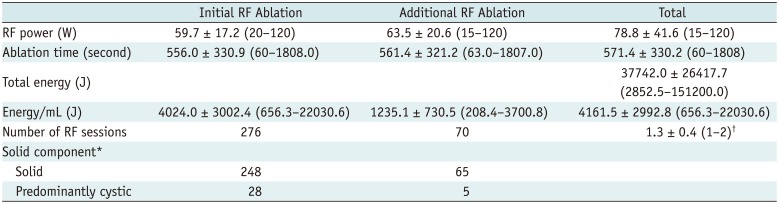
Table 3
Outcomes for 276 Benign Thyroid Nodules after RF ablation
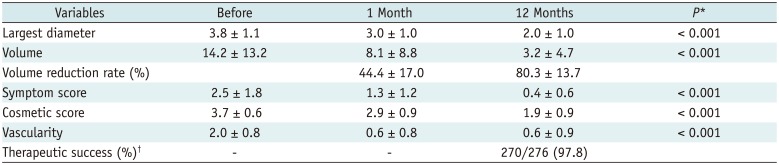
Table 4
Multiple Linear Regression Analysis of Factors Independently Predictive of Volume Reduction
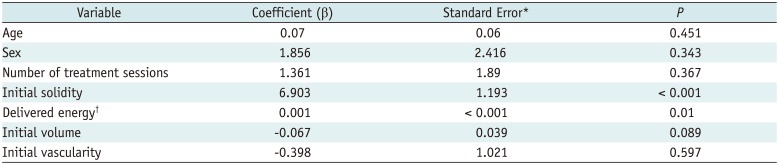
Table 5
Complications and Side Effects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download