Abstract
In 2010, the Asian Society of Cardiovascular Imaging (ASCI) provided recommendations for cardiac CT and MRI, and this document reflects an update of the 2010 ASCI appropriate use criteria (AUC). In 2016, the ASCI formed a new working group for revision of AUC for noninvasive cardiac imaging. A major change that we made in this document is the rating of various noninvasive tests (exercise electrocardiogram, echocardiography, positron emission tomography, single-photon emission computed tomography, radionuclide imaging, cardiac magnetic resonance, and cardiac computed tomography/angiography), compared side by side for their applications in various clinical scenarios. Ninety-five clinical scenarios were developed from eight selected pre-existing guidelines and classified into four sections as follows: 1) detection of coronary artery disease, symptomatic or asymptomatic; 2) cardiac evaluation in various clinical scenarios; 3) use of imaging modality according to prior testing; and 4) evaluation of cardiac structure and function. The clinical scenarios were scored by a separate rating committee on a scale of 1–9 to designate appropriate use, uncertain use, or inappropriate use according to a modified Delphi method. Overall, the AUC ratings for CT were higher than those of previous guidelines. These new AUC provide guidance for clinicians choosing among available testing modalities for various cardiac diseases and are also unique, given that most previous AUC for noninvasive imaging include only one imaging technique. As cardiac imaging is multimodal in nature, we believe that these AUC will be more useful for clinical decision making.
Noninvasive cardiac imaging procedures provide essential information for the detection, diagnosis, and management of cardiovascular diseases and serve a vital role in risk assessment and clinical decision making. The range of diagnostic tools used to evaluate cardiovascular disease has expanded over the past decade; in particular, computed tomography (CT) and magnetic resonance (MR) have emerged as alternatives to echocardiography, exercise electrocardiography (ECG), and invasive angiography.
Guidelines developed in the United States and Europe are often not applicable in Asian countries because of differences in healthcare systems, medical expenses, body habitus, and disease prevalence between Asian and Western countries. For this reason, the Asian Society of Cardiovascular Imaging (ASCI) separately developed ASCI appropriate use criteria (AUC) for cardiac CT and MR in 2010. Since the introduction of ASCI AUC in 2010, there has been further accumulation of scientific evidence and advances in imaging technology.
Currently, there are many guidelines published by different cardiovascular societies led by various expert groups; as a consequence, there are various AUC for different modalities and diseases and from different countries. The American College of Cardiology Foundation (ACCF), along with key specialty and subspecialty societies, published AUC for cardiac CT and cardiac MR (CMR) in 2006 (1). In 2010, AUC for CT were published by the Society of Cardiovascular Computed Tomography (SCCT) as well as the ASCI (23). More recently, in 2015, the Korean Society of Radiology (KSR) also published AUC for CT (4). AUC for CMR were also published by the ASCI in 2010 (5) and by the KSR in 2015 (6). As for radionuclide imaging (RNI), the ACCF, along with key specialty and subspecialty societies, published AUC in 2009 (7), although they were limited to coronary artery disease only. Moreover, the ACCF and key specialty and subspecialty societies published AUC for echocardiography in 2011 (8). There are also many guidelines for specific clinical scenarios such as for appropriate utilization of various cardiac imaging modalities in the diagnosis and treatment of heart failure (910), hypertrophic cardiomyopathy (11), and stable ischemic heart disease (1213). However, few guidelines encompass various clinical scenarios, and to the best of our knowledge, there have been no multimodality AUC in Asia. Thus, we believe that multimodality AUC for different clinical scenarios would be relevant as an update for the previous ASCI CT and MR guidelines. The purpose of this document is to delineate the appropriate use of various noninvasive testing modalities for the diagnosis and evaluation of heart disease, as well as to update the previous 2010 ASCI AUC for cardiac CT and MR.
In March 2016, the need for an update of ASCI AUC was discussed at the ASCI Administration office-presidential office meeting. A steering committee was appointed among the board of directors in order to establish plans and a budget for the AUC update. In addition, a writing committee was to be appointed from the Korean members, a rating committee to be comprised of major speakers and researchers among the ASCI members, and a review committee to be comprised of previous presidents, vicepresidents, and congress presidents of the ASCI. The plan was approved at the annual ASCI meeting held in Singapore in August 2016.
The working group consisted of the following committees:
Young Jin Kim, Jeong A Kim, Sung Mok Kim, Kyongmin Sarah Beck, Hwan Seok Yong, Dong Hyun Yang, Yoo Jin Hong
The number of technical panel members on the rating committee from each country was decided by the working group according to participation in the ASCI executive committee and ASCI annual meetings, as well as academic credentials of the investigators in the field. Thirty-three experts were nominated for the technical panel, taking into account the members' nationalities and areas of expertise, and all were approved by the working group in consensus. Twenty-two of the 33 technical panel members responded and participated in the consensus process according to the modified Delphi method.
Sung A Chang (Korea, Cardiology), Jin-Ho Choi (Korea, Cardiology), Sang-Chol Lee (Korea, Cardiology), Seung-Pyo Lee (Korea, Cardiology), Yeonyee Yoon (Korea, Cardiology), Kakuya Kitagawa (Japan, Radiology and Cardiology), Keiichiro Yoshinaga (Japan, Nuclear Medicine), Won Jun Kang (Korea, Nuclear Medicine), Jin Chul Paeng (Korea, Nuclear Medicine), Stephen Cheung (Hong Kong, Radiology), Akira Kurata (Japan, Radiology), Makoto Takamiya (Japan, Radiology), Whal Lee (Korea, Radiology), Sang IL Choi (Korea, Radiology), Eun Ju Chun (Korea, Radiology), Joon-Won Kang (Korea, Radiology), Sung Min Ko (Korea, Radiology), Jung Im Jung (Korea, Radiology), Ming-Ting Wu (Taiwan, Radiology), Wen-Yih Tseng (Taiwan, Radiology), Wen-Jeng Lee (Taiwan, Radiology), Masahiro Jinzaki (Japan, Radiology)
Because the ASCI CT and MR AUC were last published in 2010, we searched online databases for guidelines on noninvasive imaging published since 2010; if such guidelines were unavailable for certain modalities, we then searched for the most recently published guidelines instead. The following online databases were searched: Ovid-Medline, Ovid-Embase, National Guideline Clearing, and Guideline International Network. For development of this consensus document, we reviewed pre-existing utilization guidelines from countries worldwide. Eight pre-existing guidelines (345678913) were finally selected for guideline adaptation: 1) ACCF cardiac radionuclide imaging guideline 2009 (7), 2) ASCI CMR guideline 2010 (5), 3) ASCI cardiac computed tomography angiography (CCTA) guideline 2010 (3), 4) ACCF echocardiography guideline 2011 (8), 5) ACCF multimodality guideline for stable ischemic heart disease 2013 (9), 6) ACCF cardiovascular imaging in heart failure 2013 (13), 7) Korean CMR guideline 2014 (6), 8) Korean CCTA guideline 2014 (4).
The key questions were developed by the writing committee. To establish the key questions, the writing committee reviewed the previously published guidelines for each imaging modality as well as multimodality guidelines for ischemic heart disease and heart failure. After collecting all of the existing clinical questions from the guidelines, the writing committee classified the questions into four sections as follows: 1) detection of coronary artery disease, symptomatic or asymptomatic; 2) cardiac evaluation in various clinical scenarios; 3) use of imaging modality according to prior testing; and 4) evaluation of cardiac structure and function. Since questions in the previous guidelines varied by imaging modality, the writing committee selected questions common to each imaging method. Questions limited to a specific imaging method were changed or combined, conforming to more general clinical situations. Each question was modified based on feedback from independent reviewers who were cardiovascular experts. Finally, the writing committee established four sections comprised of 95 clinical scenarios for various noninvasive modalities.
The appropriateness use criteria were defined with three ratings: appropriate (A), uncertain (U), and inappropriate (I). A questionnaire was emailed to the rating committee and then collected by the ASCI office after completion. The questionnaires were collected between December 2016 and February 2017 (22 of the 33 nominated members of the rating committee responded to the survey).
The questionnaire had four sections with 95 clinical scenarios. A total of two rounds of consensus survey were conducted; for each round, the appropriateness of utilization was categorized with a 9-point response scale: 1–3 points as I, 4–6 points as U, and 7–9 points as A. For each round, different imaging modalities were separately scored for their appropriateness in a given scenario. When more than 50% of the panelists agreed on a category, the panel was considered to have reached a consensus for that particular clinical scenario. The questionnaire form included appropriateness criteria from other guidelines for each category and each noninvasive test modality (exercise ECG, echocardiography, positron emission tomography, single-photon emission computed tomography [SPECT], RNI, CMR, and CCTA), the 9-point response scale, and space for additional comments. In the second round, the median scores from the previous round and the scores originally given by the answering panelist were shown for the questions for which agreement had not been reached. The questions with agreement reached in the previous round were not shown in the following round.
Of the 95 clinical scenarios-comprised of a total of 455 questions for different modalities-sent for the first round, consensus was reached on all modalities in 42 scenarios (197 questions). Of the other 53 scenarios (258 questions), 86 questions for which consensus was not reached were sent for the second round. The results of the consensus voting are included in the Supplementary (in the online-only Data Supplement).
When interpreting the score, several specific assumptions should be considered. Presumably, each test is performed in compliance with published criteria for quality cardiac diagnostic testing, locally available, and interpreted by experts who are qualified to do so. For exercise ECG, it should be assumed that the patient can exercise to a symptomatic endpoint or 85% of their age-predicted maximal heart rate. For echocardiography, SPECT, and CMR in evaluation of coronary artery disease, it is assumed that pharmacological stress test is performed to identify the presence of myocardial ischemia. Each modality has inherent risks such as radiation exposure, contrast sensitivity, and interpretation error. It is assumed that each modality should be chosen after weighing the risks and benefits in the specific clinical scenario. It should be assumed that CCTA and SPECT are performed using contemporary dose-saving techniques conforming to the As Low As Reasonably Achievable (ALARA) principle. For reasonable use of cardiovascular modality, the As High As Reasonably Achievable (AHARA) principle was considered.
The review committee, consisting of past-presidents, vice-presidents, and congress presidents, reviewed the AUC selected by consensus.
The development of the current AUC was funded by the ASCI. However, the activities of the writing committee, the rating committee, and the review committee were independent of one another, and none of the three committees were influenced in any way by any of the funding for guideline development.
These guidelines should be revised as needed, following advances in technology, changes in the healthcare environment, and further accumulation of scientific evidence.
These new ASCI multimodality AUC were developed in order to reflect the current status of noninvasive cardiac imaging in Asia. In the current document, we present a synthesis of clinical experience for all commonly employed noninvasive imaging procedures for diagnosis of various cardiovascular diseases.
This document covers the same or similar clinical scenarios as the prior ASCI AUC for CT and MR and other modality guidelines for individual procedures. Overall, the AUC ratings for CT are higher than those of the previous guidelines. This difference might be attributable to advances in CT technology, which have resulted in reduced radiation exposure and more accurate evaluation of small structures with improvement in temporal resolution. In addition, wide availability of CT in Asian countries compared to CMR, which is less accessible, could be another cause of the improved rating of CT.
These rating differences might also reflect the changing practice environment and evolution in cumulative clinical experience with these procedures, as well as maturation of the field since publication of the original documents.
These new AUC are intended to provide guidance for clinicians when choosing among available testing modalities for various cardiac diseases. Each test was rated individually for each scenario based on the quality of the published evidence as well as the expert opinion of the rating panel. In the absence of robust evidence of comparative effectiveness, a comparative rating approach would be both premature and misleading. In addition, a larger number of radiologists in the writing and rating committees might have resulted in somewhat skewed ratings for certain modalities. Thus, although these ratings reflect existing evidence-based practice supplemented by expert consensus, further research is needed to identify not only when to use any given modality, but also when to favor one over another.
Notes
References
1. Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006; 48:1475–1497. PMID: 17010819.
2. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/ SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010; 4:407. PMID: 21232696.
3. ASCI CCT & CMR Guideline Working Group. Tsai IC, Choi BW, Chan C, Jinzaki M, Kitagawa K, et al. ASCI 2010 appropriateness criteria for cardiac computed tomography: a report of the Asian Society of Cardiovascular Imaging Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging Guideline Working Group. Int J Cardiovasc Imaging. 2010; 26(Suppl 1):1–15.

4. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285. PMID: 25741189.

5. ASCI CCT, Kitagawa K, Choi BW, Chan C, Jinzaki M, Tsai IC, et al. ASCI 2010 appropriateness criteria for cardiac magnetic resonance imaging: a report of the Asian Society of Cardiovascular Imaging cardiac computed tomography and cardiac magnetic resonance imaging guideline working group. Int J Cardiovasc Imaging. 2010; 26(Suppl 2):173–186.

6. Yoon YE, Hong YJ, Kim HK, Kim JA, Na JO, Yang DH, et al. 2014 Korean guidelines for appropriate utilization of cardiovascular magnetic resonance imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean J Radiol. 2014; 15:659–688. PMID: 25469078.

7. Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/ SNM 2009 Appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009; 53:2201–2229. PMID: 19497454.
8. American College of Cardiology Foundation Appropriate Use Criteria Task Force. American Society of Echocardiography. American Heart Association. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society. ACCF/ASE/AHA/ASNC/HFSA/HRS/ SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011; 57:1126–1166. PMID: 21349406.
9. Patel MR, White RD, Abbara S, Bluemke DA, Herfkens RJ, Picard M, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: a joint report of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Foundation Appropriate Use Criteria Task Force. J Am Coll Cardiol. 2013; 61:2207–2231. PMID: 23500216.
10. White RD, Patel MR, Abbara S, Bluemke DA, Herfkens RJ, Picard M, et al. 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: an executive summary: a joint report of the ACR Appropriateness Criteria® Committee and the ACCF Appropriate Use Criteria Task Force. J Am Coll Radiol. 2013; 10:493–500. PMID: 23827001.
11. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in Collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 58:e212–e260. PMID: 22075469.
12. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/ STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J AM Coll Cardiol. 2012; 60:e444–e164.
13. Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014; 63:380–406. PMID: 24355759.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3348/kjr.2017.18.6.871.
Table 1
Symptomatic: Non-Acute Chest Pain Suspected of Stable Coronary Artery Disease
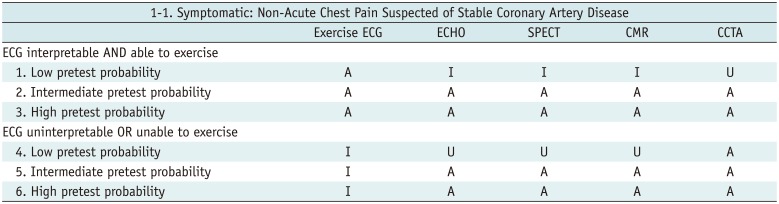
Table 2
Symptomatic: Acute Chest Pain Suspected of Acute Coronary Syndrome
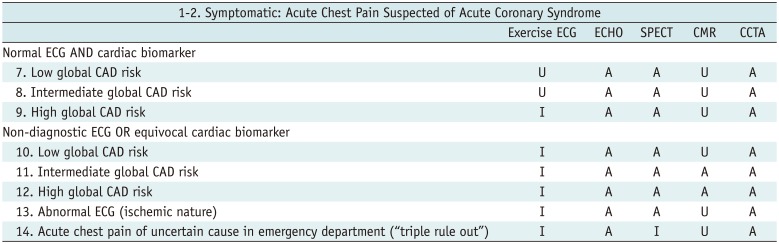
Table 3
Asymptomatic (1)
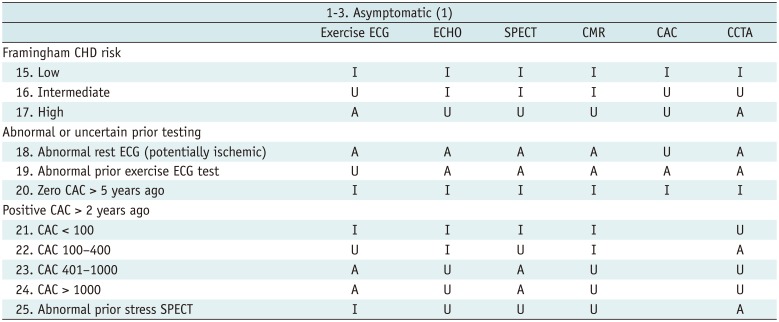
Table 4
Asymptomatic (2): Post-Revascularization (PCI or CABG)
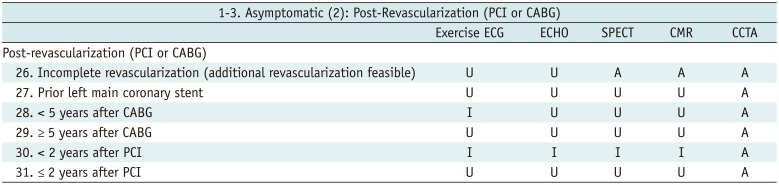
Table 5
Newly Developed or Suspected Heart Failure
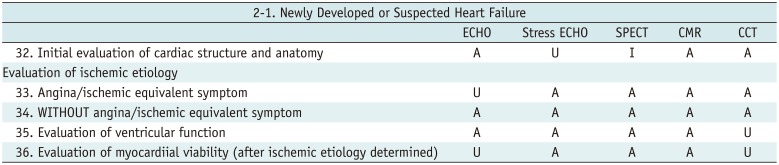
Table 6
Cardiac Evaluation Prior to Surgery
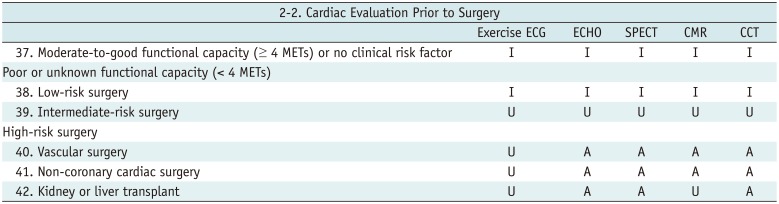
Table 7
Evaluation of Arrhythmia or Syncope without Ischemic Etiology
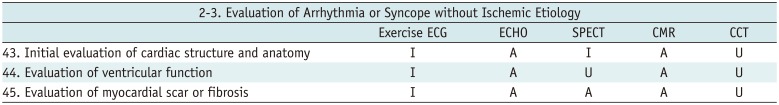
Table 8
Coronary Revascularization
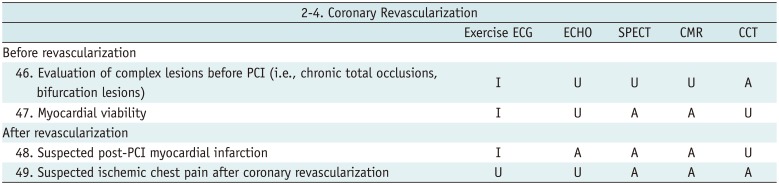
Table 9
Kawasaki Disease
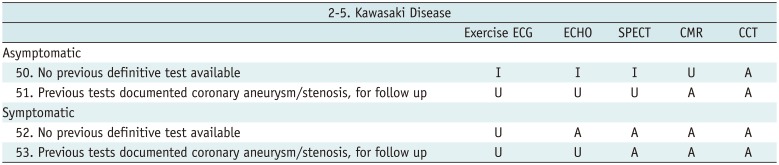
Table 10
Prior Exercise ECG
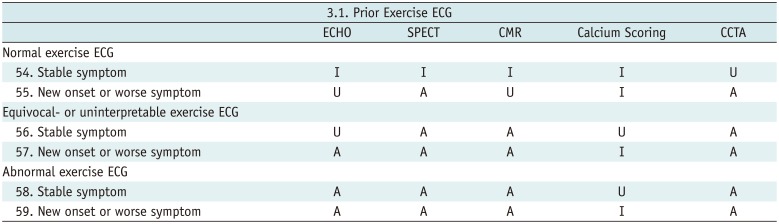
Table 11
Prior SPECT
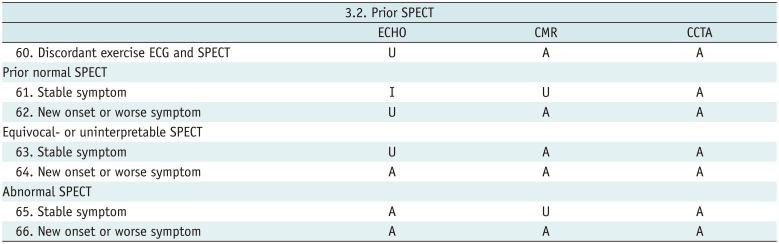
Table 12
Prior CCTA
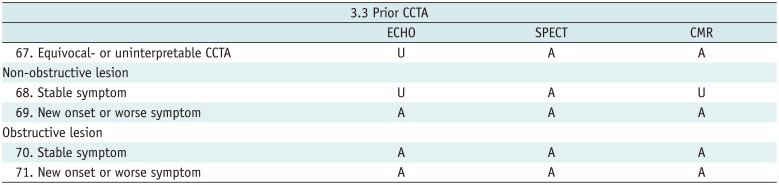
Table 13
Congenital Heart Disease
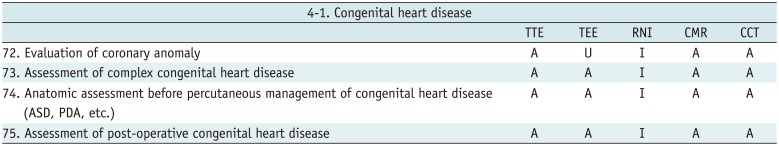
Table 14
Valvular Heart Disease (Native Valve AND Prosthetic Valve)
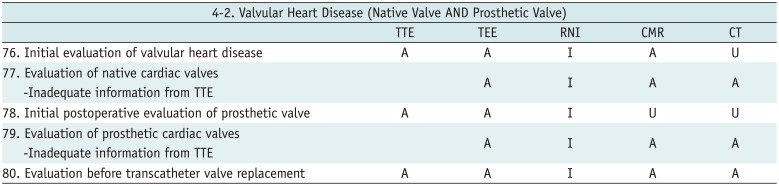
Table 15
Cardiomyopathy (after Ischemic Etiology Ruled Out)
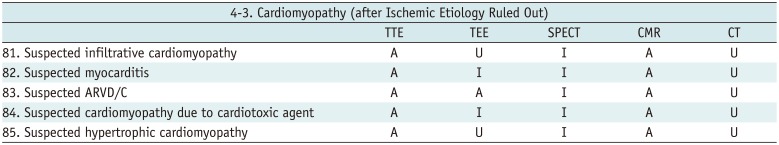
Table 16
Electrophysiology Study, Ablation, ICD/CRT
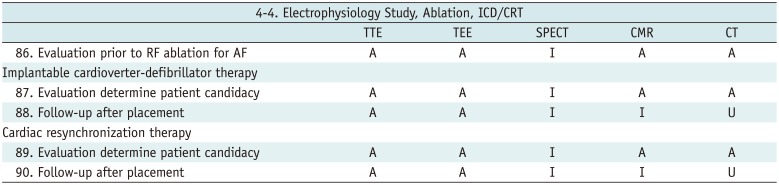
Table 17
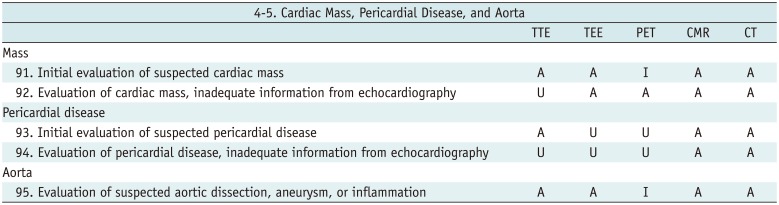
Cardiac Mass, Pericardial Disease, and Aorta




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download